18F-labeled mini-PEG spacered RGD dimer (18F-FPRGD2): synthesis and microPET imaging of alphavbeta3 integrin expression
- PMID: 17492285
- PMCID: PMC4167588
- DOI: 10.1007/s00259-007-0427-0
18F-labeled mini-PEG spacered RGD dimer (18F-FPRGD2): synthesis and microPET imaging of alphavbeta3 integrin expression
Abstract
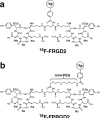
Purpose: We have previously reported that (18)F-FB-E[c(RGDyK)](2) ((18)F-FRGD2) allows quantitative PET imaging of integrin alpha(v)beta(3) expression. However, the potential clinical translation was hampered by the relatively low radiochemical yield. The goal of this study was to improve the radiolabeling yield, without compromising the tumor targeting efficiency and in vivo kinetics, by incorporating a hydrophilic bifunctional mini-PEG spacer.
Methods: (18)F-FB-mini-PEG-E[c(RGDyK)](2) ((18)F-FPRGD2) was synthesized by coupling N-succinimidyl-4-(18)F-fluorobenzoate ((18)F-SFB) with NH(2)-mini-PEG-E[c(RGDyK)](2) (denoted as PRGD2). In vitro receptor binding affinity, metabolic stability, and integrin alpha(v)beta(3) specificity of the new tracer (18)F-FPRGD2 were assessed. The diagnostic value of (18)F-FPRGD2 was evaluated in subcutaneous U87MG glioblastoma xenografted mice and in c-neu transgenic mice by quantitative microPET imaging studies.
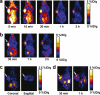
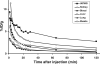
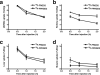
Results: The decay-corrected radiochemical yield based on (18)F-SFB was more than 60% with radiochemical purity of >99%. (18)F-FPRGD2 had high receptor binding affinity, metabolic stability, and integrin alpha(v)beta(3)-specific tumor uptake in the U87MG glioma xenograft model comparable to those of (18)F-FRGD2. The kidney uptake was appreciably lower for (18)F-FPRGD2 compared with (18)F-FRGD2 [2.0 +/- 0.2%ID/g for (18)F-FPRGD2 vs 3.0 +/- 0.2%ID/g for (18)F-FRGD2 at 1 h post injection (p.i.)]. The uptake in all the other organs except the urinary bladder was at background level. (18)F-FPRGD2 also exhibited excellent tumor uptake in c-neu oncomice (3.6 +/- 0.1%ID/g at 30 min p.i.).
Conclusion: Incorporation of a mini-PEG spacer significantly improved the overall radiolabeling yield of (18)F-FPRGD2. (18)F-FPRGD2 also had reduced renal uptake and similar tumor targeting efficacy as compared with (18)F-FRGD2. Further testing and clinical translation of (18)F-FPRGD2 are warranted.
Figures

References
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. - PubMed
-
- Cai W, Chen X. Anti-angiogenic cancer therapy based on integrin αvβ3 antagonism. Anti-Cancer Agents Med Chem. 2006;6:407–28. - PubMed
-
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–71. - PubMed
-
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. - PubMed
-
- Haubner R, Wester H-J, Weber WA, Mang C, Ziegler SI, Goodman SL, et al. Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001;61:1781–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

