Phospholipase C beta3 is a key component in the Gbetagamma/PKCeta/PKD-mediated regulation of trans-Golgi network to plasma membrane transport
- PMID: 17492941
- PMCID: PMC1948997
- DOI: 10.1042/BJ20070359
Phospholipase C beta3 is a key component in the Gbetagamma/PKCeta/PKD-mediated regulation of trans-Golgi network to plasma membrane transport
Abstract
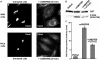
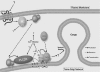
The requirement of DAG (diacylglycerol) to recruit PKD (protein kinase D) to the TGN (trans-Golgi network) for the targeting of transport carriers to the cell surface, has led us to a search for new components involved in this regulatory pathway. Previous findings reveal that the heterotrimeric Gbetagamma (GTP-binding protein betagamma subunits) act as PKD activators, leading to fission of transport vesicles at the TGN. We have recently shown that PKCeta (protein kinase Ceta) functions as an intermediate member in the vesicle generating pathway. DAG is capable of activating this kinase at the TGN, and at the same time is able to recruit PKD to this organelle in order to interact with PKCeta, allowing phosphorylation of PKD's activation loop. The most qualified candidates for the production of DAG at the TGN are PI-PLCs (phosphatidylinositol-specific phospholipases C), since some members of this family can be directly activated by Gbetagamma, utilizing PtdIns(4,5)P2 as a substrate, to produce the second messengers DAG and InsP3. In the present study we show that betagamma-dependent Golgi fragmentation, PKD1 activation and TGN to plasma membrane transport were affected by a specific PI-PLC inhibitor, U73122 [1-(6-{[17-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-1H-pyrrole-2,5-dione]. In addition, a recently described PI-PLC activator, m-3M3FBS [2,4,6-trimethyl-N-(m-3-trifluoromethylphenyl)benzenesulfonamide], induced vesiculation of the Golgi apparatus as well as PKD1 phosphorylation at its activation loop. Finally, using siRNA (small interfering RNA) to block several PI-PLCs, we were able to identify PLCbeta3 as the sole member of this family involved in the regulation of the formation of transport carriers at the TGN. In conclusion, we demonstrate that fission of transport carriers at the TGN is dependent on PI-PLCs, specifically PLCbeta3, which is necessary to activate PKCeta and PKD in that Golgi compartment, via DAG production.
Figures



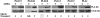

References
-
- Takizawa P. A., Yucel J. K., Veit B., Faulkner D. J., Deerinck T., Soto G., Ellisman M., Malhotra V. Complete vesiculation of Golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone. Cell. 1993;73:1079–1090. - PubMed
-
- Jamora C., Takizawa P. A., Zaarour R. F., Denesvre C., Faulkner D. J., Malhotra V. Regulation of Golgi structure through heterotrimeric G proteins. Cell. 1997;91:617–626. - PubMed
-
- Jamora C., Yamanouye N., Van Lint J., Laudenslager J., Vandenheede J. R., Faulkner D. J., Malhotra V. Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98:59–68. - PubMed
-
- Liljedahl M., Maeda Y., Colanzi A., Ayala I., Van Lint J., Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

