Molecular analysis of the sea anemone toxin Av3 reveals selectivity to insects and demonstrates the heterogeneity of receptor site-3 on voltage-gated Na+ channels
- PMID: 17492942
- PMCID: PMC1948988
- DOI: 10.1042/BJ20070233
Molecular analysis of the sea anemone toxin Av3 reveals selectivity to insects and demonstrates the heterogeneity of receptor site-3 on voltage-gated Na+ channels
Abstract
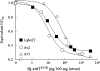
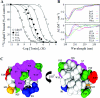
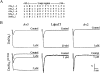
Av3 is a short peptide toxin from the sea anemone Anemonia viridis shown to be active on crustaceans and inactive on mammals. It inhibits inactivation of Na(v)s (voltage-gated Na+ channels) like the structurally dissimilar scorpion alpha-toxins and type I sea anemone toxins that bind to receptor site-3. To examine the potency and mode of interaction of Av3 with insect Na(v)s, we established a system for its expression, mutagenized it throughout, and analysed it in toxicity, binding and electrophysiological assays. The recombinant Av3 was found to be highly toxic to blowfly larvae (ED50=2.65+/-0.46 pmol/100 mg), to compete well with the site-3 toxin LqhalphaIT (from the scorpion Leiurus quinquestriatus) on binding to cockroach neuronal membranes (K(i)=21.4+/-7.1 nM), and to inhibit the inactivation of Drosophila melanogaster channel, DmNa(v)1, but not that of mammalian Na(v)s expressed in Xenopus oocytes. Moreover, like other site-3 toxins, the activity of Av3 was synergically enhanced by ligands of receptor site-4 (e.g. scorpion beta-toxins). The bioactive surface of Av3 was found to consist mainly of aromatic residues and did not resemble any of the bioactive surfaces of other site-3 toxins. These analyses have portrayed a toxin that might interact with receptor site-3 in a different fashion compared with other ligands of this site. This assumption was corroborated by a D1701R mutation in DmNa(v)1, which has been shown to abolish the activity of all other site-3 ligands, except Av3. All in all, the present study provides further evidence for the heterogeneity of receptor site-3, and raises Av3 as a unique model for design of selective anti-insect compounds.
Figures
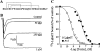

References
-
- Catterall W. A. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. - PubMed
-
- Ulbricht W. Sodium channel inactivation: molecular determinants and modulation. Physiol. Rev. 2005;85:1271–1301. - PubMed
-
- Blumenthal K. M., Seibert A. L. Voltage-gated sodium channel toxins: poison, probes, and future promise. Cell. Biochem. Biophys. 2003;38:215–238. - PubMed
-
- Catterall W. A., Beress L. Sea anemone toxin and scorpion toxin share a common receptor site associated with the action potential sodium ionophore. J. Biol. Chem. 1978;253:7393–7396. - PubMed
-
- Little M. J., Zappia C., Gilles N., Connor M., Tyler M. I., Martin-Eauclaire M. F., Gordon D., Nicholson G. M. δ-Atracotoxins from Australian funnel-web spiders compete with scorpion α-toxin binding but differentially modulate alkaloid toxin activation of voltage-gated sodium channels. J. Biol. Chem. 1998;273:27076–27083. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

