Structural principles of tau and the paired helical filaments of Alzheimer's disease
- PMID: 17493042
- PMCID: PMC8095506
- DOI: 10.1111/j.1750-3639.2007.00053.x
Structural principles of tau and the paired helical filaments of Alzheimer's disease
Abstract
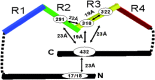
Tau, a major microtubule-associated protein in brain, forms abnormal fibers in Alzheimer's disease and several other neurodegenerative diseases. Tau is highly soluble and adopts a natively unfolded structure in solution. In the paired helical filaments of Alzheimer's disease, small segments of tau adopt a beta-conformation and interact with other tau molecules. In the filament core, the microtubule-binding repeat region of tau has a cross-beta structure, while the rest of the protein retains its largely unfolded structure and gives rise to the fuzzy coat of the filaments.
Figures




References
-
- Alzheimer A (1907) Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychia 64:146–148.
-
- Andorfer C, Kress Y, Espinoza M, De Silva R, Tucker KL, Barde YA, Duff K, Davies P (2003) Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem 86:582–590. - PubMed
-
- Andreadis A (2005) Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim Biophys Acta 1739:91–103. - PubMed
-
- Arai T, Guo JP, McGeer PL (2005) Proteolysis of non‐phosphorylated and phosphorylated tau by thrombin. J Biol Chem 280:5145–5153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

