distal antenna and distal antenna-related function in the retinal determination network during eye development in Drosophila
- PMID: 17493605
- PMCID: PMC1986786
- DOI: 10.1016/j.ydbio.2007.04.006
distal antenna and distal antenna-related function in the retinal determination network during eye development in Drosophila
Abstract
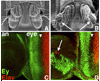
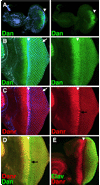
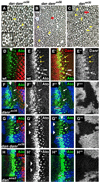
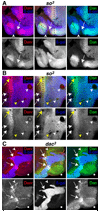
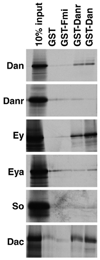
Drosophila eye specification occurs through the activity of the retinal determination (RD) network, which includes the Eyeless (Ey), Eyes absent (Eya), Sine oculis (So) and Dachshund (Dac) transcription factors. Based on their abilities to transform antennal precursors towards an eye fate, the distal antenna (dan) and distal antenna-related (danr) genes encode two new RD factors. Dan and Danr are probable transcription factors localized in nuclei of eye precursors and differentiating eye tissue. Loss-of-function single and double dan/danr mutants have small, rough eyes, indicating a requirement for wild-type eye development. In addition, dan and danr participate in the transcriptional hierarchy that controls expression of RD genes, and Dan and Danr interact physically and genetically with Ey and Dac. Eye specification culminates in differentiation of ommatidia through the activities of the proneural gene atonal (ato) in the founding R8 photoreceptor and Egfr signaling in additional photoreceptors. Danr expression overlaps with Ato during R8 specification, and Dan and Danr regulate Ato expression and are required for normal R8 induction and differentiation. These data demonstrate a role for Dan and Danr in eye development and provide a link between eye specification and differentiation.
Figures


References
-
- Baker NE, Rubin GM. Effect on eye development of dominant mutations in Drosophila homologue of the EGF receptor. Nature. 1989;340:150–153. - PubMed
-
- Baker NE, Rubin GM. Ellipse mutations in the Drosophila homologue of the EGF receptor affect pattern formation, cell division, and cell death in eye imaginal discs. Dev. Biol. 1992;150:381–396. - PubMed
-
- Baker NE, Yu S, Han D. Evolution of proneural atonal expression during distinct regulatory phases in the developing Drosophila eye. Curr. Biol. 1996;6:1290–1301. - PubMed
-
- Baker NE, Yu SY. Proneural function of neurogenic genes in the developing Drosophila eye. Curr. Biol. 1997;7:122–132. - PubMed
-
- Baonza A, Casci T, Freeman M. A primary role for the epidermal growth factor receptor in ommatidial spacing in the Drosophila eye. Curr. Biol. 2001;11:396–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

