Conditional deletion of beta1-integrin from the developing lens leads to loss of the lens epithelial phenotype
- PMID: 17493607
- PMCID: PMC1950782
- DOI: 10.1016/j.ydbio.2007.04.004
Conditional deletion of beta1-integrin from the developing lens leads to loss of the lens epithelial phenotype
Abstract
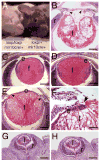
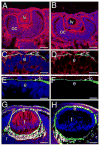
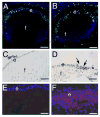
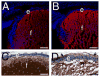
Beta1-integrins are cell surface receptors that participate in sensing the cell's external environment. We used the Cre-lox system to delete beta1-integrin in all lens cells as the lens vesicle transitions into the lens. Adult mice lacking beta1-integrin in the lens are microphthalmic due to apoptosis of the lens epithelium and neonatal disintegration of the lens fibers. The first morphological alterations in beta1-integrin null lenses are seen at 16.5 dpc when the epithelium becomes disorganized and begins to upregulate the fiber cell markers beta- and gamma-crystallins, the transcription factors cMaf and Prox1 and downregulate Pax6 levels demonstrating that beta1-integrin is essential to maintain the lens epithelial phenotype. Furthermore, beta1-integrin null lens epithelial cells upregulate the expression of alpha-smooth muscle actin and nuclear Smad4 and downregulate Smad6 suggesting that beta1-integrin may brake TGFbeta family signaling leading to epithelial-mesenchymal transitions in the lens. In contrast, beta1-integrin null lens epithelial cells show increased E-cadherin immunoreactivity which supports the proposed role of beta1-integrins in mediating complete EMT in response to TGFbeta family members. Thus, beta1-integrin is required to maintain the lens epithelial phenotype and block inappropriate activation of some aspects of the lens fiber cell differentiation program.
Figures




Similar articles
-
Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation.Cells Tissues Organs. 2005;179(1-2):43-55. doi: 10.1159/000084508. Cells Tissues Organs. 2005. PMID: 15942192 Review.
-
β1-integrin controls cell fate specification in early lens development.Differentiation. 2016 Oct-Nov;92(4):133-147. doi: 10.1016/j.diff.2016.08.002. Epub 2016 Sep 3. Differentiation. 2016. PMID: 27596755 Free PMC article.
-
Beta-1 integrin is important for the structural maintenance and homeostasis of differentiating fiber cells.Int J Biochem Cell Biol. 2014 May;50:132-45. doi: 10.1016/j.biocel.2014.02.021. Epub 2014 Mar 4. Int J Biochem Cell Biol. 2014. PMID: 24607497 Free PMC article.
-
Overexpression of PAX6(5a) in lens fiber cells results in cataract and upregulation of (alpha)5(beta)1 integrin expression.J Cell Sci. 2000 Sep;113 ( Pt 18):3173-85. doi: 10.1242/jcs.113.18.3173. J Cell Sci. 2000. PMID: 10954416
-
An overview of epithelio-mesenchymal transformation.Acta Anat (Basel). 1995;154(1):8-20. doi: 10.1159/000147748. Acta Anat (Basel). 1995. PMID: 8714286 Review.
Cited by
-
Molecular characterization of the human lens epithelium-derived cell line SRA01/04.Exp Eye Res. 2019 Nov;188:107787. doi: 10.1016/j.exer.2019.107787. Epub 2019 Aug 31. Exp Eye Res. 2019. PMID: 31479653 Free PMC article.
-
Integrins in lens development and disease.Exp Eye Res. 2009 Feb;88(2):216-25. doi: 10.1016/j.exer.2008.06.020. Epub 2008 Jul 11. Exp Eye Res. 2009. PMID: 18671967 Free PMC article. Review.
-
Deletion of β1-integrin in collecting duct principal cells leads to tubular injury and renal medullary fibrosis.Am J Physiol Renal Physiol. 2017 Oct 1;313(4):F1026-F1037. doi: 10.1152/ajprenal.00038.2017. Epub 2017 Jul 12. Am J Physiol Renal Physiol. 2017. PMID: 28701310 Free PMC article.
-
Dual function of Yap in the regulation of lens progenitor cells and cellular polarity.Dev Biol. 2014 Feb 15;386(2):281-90. doi: 10.1016/j.ydbio.2013.12.037. Epub 2013 Dec 31. Dev Biol. 2014. PMID: 24384391 Free PMC article.
-
Human lens epithelial cells induce the inflammatory response when placed into the lens capsular bag model of posterior capsular opacification.Mol Vis. 2024 Oct 8;30:348-367. eCollection 2024. Mol Vis. 2024. PMID: 39959166 Free PMC article.
References
-
- Barbour W, Saika S, Miyamoto T, Ohkawa K, Utsunomiya H, Ohnishi Y. Expression patterns of beta1-related alpha integrin subunits in murine lens during embryonic development and wound healing. Curr Eye Res. 2004;29:1–10. - PubMed
-
- Bassnett S, Kuszak JR, Reinisch L, Brown HG, Beebe DC. Intercellular communication between epithelial and fiber cells of the eye lens. J Cell Sci. 1994;107(Pt 4):799–811. - PubMed
-
- Bassnett S, Missey H, Vucemilo I. Molecular architecture of the lens fiber cell basal membrane complex. J Cell Sci. 1999;112:2155–2165. - PubMed
-
- Belkin AM, Stepp MA. Integrins as receptors for laminins. Microsc Res Tech. 2000;51:280–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

