Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation
- PMID: 17493609
- PMCID: PMC1974901
- DOI: 10.1016/j.yexcr.2007.03.036
Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation
Abstract
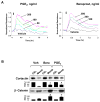
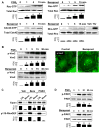
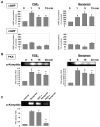
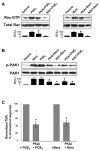
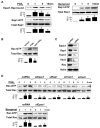
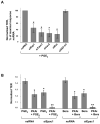
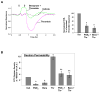
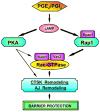
Prostaglandin E(2) (PGE(2)) and prostacyclin are lipid mediators produced by cyclooxygenase and implicated in the regulation of vascular function, wound repair, inflammatory processes, and acute lung injury. Although protective effects of these prostaglandins (PGs) are associated with stimulation of intracellular cAMP production, the crosstalk between cAMP-activated signal pathways in the regulation of endothelial cell (EC) permeability is not well understood. We studied involvement of cAMP-dependent kinase (PKA), cAMP-Epac-Rap1 pathway, and small GTPase Rac in the PGs-induced EC barrier protective effects and cytoskeletal remodeling. PGE(2) and PGI(2) synthetic analog beraprost increased transendothelial electrical resistance and decreased dextran permeability, enhanced peripheral F-actin rim and increased intercellular adherens junction areas reflecting EC barrier-protective response. Furthermore, beraprost dramatically attenuated thrombin-induced Rho activation, MLC phosphorylation and EC barrier dysfunction. In vivo, beraprost attenuated lung barrier dysfunction induced by high tidal volume mechanical ventilation. Both PGs caused cAMP-mediated activation of PKA-, Epac/Rap1- and Tiam1/Vav2-dependent pathways of Rac1 activation and EC barrier regulation. Knockdown of Epac, Rap1, Rac-specific exchange factors Tiam1 and Vav2 using siRNA approach, or inhibition of PKA activity decreased Rac1 activation and PG-induced EC barrier enhancement. Thus, our results show that barrier-protective effects of PGE(2) and prostacyclin on pulmonary EC are mediated by PKA and Epac/Rap pathways, which converge on Rac activation and lead to enhancement of peripheral actin cytoskeleton and adherens junctions. These mechanisms may mediate protective effects of PGs against agonist-induced lung vascular barrier dysfunction in vitro and against mechanical stress-induced lung injury in vivo.
Figures



References
-
- Ricard JD, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Eur Respir J Suppl. 2003;42:2s–9s. - PubMed
-
- Matthay MA, Zimmerman GA, Esmon C, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167:1027–35. - PubMed
-
- Howard LS, Morrell NW. New therapeutic agents for pulmonary vascular disease. Paediatr Respir Rev. 2005;6:285–91. - PubMed
-
- Ueno Y, Koike H, Annoh S, et al. Anti-inflammatory effects of beraprost sodium, a stable analogue of PGI2, and its mechanisms. Prostaglandins. 1997;53:279–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

