Cell cycle arrest and apoptosis induced by the coronavirus infectious bronchitis virus in the absence of p53
- PMID: 17493653
- PMCID: PMC7103336
- DOI: 10.1016/j.virol.2007.04.015
Cell cycle arrest and apoptosis induced by the coronavirus infectious bronchitis virus in the absence of p53
Abstract
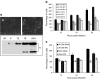
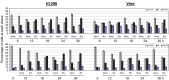
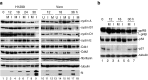
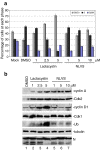
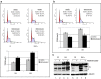
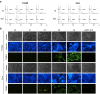
Manipulation of the cell cycle and induction of apoptosis are two common strategies used by many viruses to regulate their infection cycles. In cells infected with coronaviruses, cell cycle perturbation and apoptosis were observed in several reports. However, little is known about how these effects are brought out, and how manipulation of the functions of host cells would influence the replication cycle of coronavirus. In this study, we demonstrate that infection with coronavirus infectious bronchitis virus (IBV) imposed a growth-inhibitory effect on cultured cells by inducing cell cycle arrest at S and G(2)/M phases in both p53-null cell line H1299 and Vero cells. This cell cycle arrest was catalyzed by the modulation of various cell cycle regulatory genes and the accumulation of hypophosphorylated RB, but was independent of p53. Proteasome inhibitors, such as lactacystin and NLVS, could bypass the IBV-induced S-phase arrest by restoring the expression of corresponding cyclin/Cdk complexes. Our data also showed that cell cycle arrest at both S- and G(2)/M-phases was manipulated by IBV for the enhancement of viral replication. In addition, apoptosis induced by IBV at late stages of the infection cycle in cultured cells was shown to be p53-independent. This conclusion was drawn based on the observations that apoptosis occurred in both IBV-infected H1299 and Vero cells, and that IBV infection did not affect the expression of p53 in host cells.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

