Genetic modulation of BDNF signaling affects the outcome of axonal competition in vivo
- PMID: 17493809
- PMCID: PMC2175069
- DOI: 10.1016/j.cub.2007.04.040
Genetic modulation of BDNF signaling affects the outcome of axonal competition in vivo
Abstract
Background: Activity-dependent competition that operates on branch stability or formation plays a critical role in shaping the pattern and complexity of axonal terminal arbors. In the mammalian central nervous system (CNS), the effect of activity-dependent competition on axon arborization and on the assembly of sensory maps is well established. However, the molecular pathways that modulate axonal-branch stability or formation in competitive environments remain unknown.
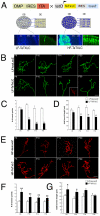
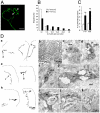
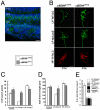
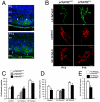
Results: We establish an in vivo axonal-competition paradigm in the mouse olfactory system by employing a genetic strategy that permits suppression of neurosecretory activity in random subsets of olfactory sensory neurons (OSNs). Long-term follow up confirmed that this genetic manipulation triggers competition by revealing a bias toward selective stabilization of active arbors and local degeneration of synaptically silent ones. By using a battery of genetically modified mouse models, we demonstrate that a decrease either in the total levels or the levels of activity-dependent secreted BDNF (due to a val66met substitution), rescues silent arbors from withering. We show that this effect may be mediated, at least in part, by p75(NTR).
Conclusions: We establish and experimentally validate a genetic in vivo axonal-competition paradigm in the mammalian CNS. By using this paradigm, we provide evidence for a specific effect of BDNF signaling on terminal-arbor pruning under competition in vivo. Our results have implications for the formation and refinement of the olfactory and other sensory maps, as well as for neuropsychiatric diseases and traits modulated by the BDNF val66met variant.
Figures


References
-
- Goda Y, Davis GW. Mechanisms of synapse assembly and disassembly. Neuron. 2003;40:243–264. - PubMed
-
- Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. - PubMed
-
- Ruthazer ES, Akerman CJ, Cline HT. Control of axon branch dynamics by correlated activity in vivo. Science. 2003;301:66–70. - PubMed
-
- Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–1026. - PubMed
-
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

