Dissociation of eye and head components of gaze shifts by stimulation of the omnipause neuron region
- PMID: 17493925
- PMCID: PMC3639481
- DOI: 10.1152/jn.00252.2007
Dissociation of eye and head components of gaze shifts by stimulation of the omnipause neuron region
Abstract
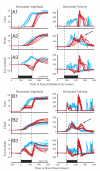
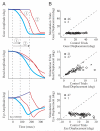
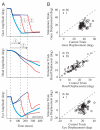
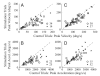
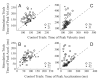
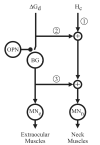
Natural movements often include actions integrated across multiple effectors. Coordinated eye-head movements are driven by a command to shift the line of sight by a desired displacement vector. Yet because extraocular and neck motoneurons are separate entities, the gaze shift command must be separated into independent signals for eye and head movement control. We report that this separation occurs, at least partially, at or before the level of pontine omnipause neurons (OPNs). Stimulation of the OPNs prior to and during gaze shifts temporally decoupled the eye and head components by inhibiting gaze and eye saccades. In contrast, head movements were consistently initiated before gaze onset, and ongoing head movements continued along their trajectories, albeit with some characteristic modulations. After stimulation offset, a gaze shift composed of an eye saccade, and a reaccelerated head movement was produced to preserve gaze accuracy. We conclude that signals subject to OPN inhibition produce the eye-movement component of a coordinated eye-head gaze shift and are not the only signals involved in the generation of the head component of the gaze shift.
Figures



Similar articles
-
Brain stem omnipause neurons and the control of combined eye-head gaze saccades in the alert cat.J Neurophysiol. 1998 Jun;79(6):3060-76. doi: 10.1152/jn.1998.79.6.3060. J Neurophysiol. 1998. PMID: 9636108
-
Action of the brain stem saccade generator during horizontal gaze shifts. I. Discharge patterns of omnidirectional pause neurons.J Neurophysiol. 1999 Mar;81(3):1284-95. doi: 10.1152/jn.1999.81.3.1284. J Neurophysiol. 1999. PMID: 10085355
-
3-Dimensional eye-head coordination in gaze shifts evoked during stimulation of the lateral intraparietal cortex.Neuroscience. 2009 Dec 15;164(3):1284-302. doi: 10.1016/j.neuroscience.2009.08.066. Epub 2009 Sep 4. Neuroscience. 2009. PMID: 19733631
-
Studies of the role of the paramedian pontine reticular formation in the control of head-restrained and head-unrestrained gaze shifts.Ann N Y Acad Sci. 2002 Apr;956:85-98. doi: 10.1111/j.1749-6632.2002.tb02811.x. Ann N Y Acad Sci. 2002. PMID: 11960796 Review.
-
Neural control of 3-D gaze shifts in the primate.Prog Brain Res. 2003;142:109-24. doi: 10.1016/s0079-6123(03)42009-8. Prog Brain Res. 2003. PMID: 12693257 Review.
Cited by
-
Dynamic and opposing adjustment of movement cancellation and generation in an oculomotor countermanding task.J Neurosci. 2013 Jun 12;33(24):9975-84. doi: 10.1523/JNEUROSCI.2543-12.2013. J Neurosci. 2013. PMID: 23761893 Free PMC article.
-
Spatiotemporal transformations for gaze control.Physiol Rep. 2020 Aug;8(16):e14533. doi: 10.14814/phy2.14533. Physiol Rep. 2020. PMID: 32812395 Free PMC article. Review.
-
Role of the primate superior colliculus in the control of head movements.J Neurophysiol. 2007 Oct;98(4):2022-37. doi: 10.1152/jn.00258.2007. Epub 2007 Jun 20. J Neurophysiol. 2007. PMID: 17581848 Free PMC article.
-
Modeling eye-head gaze shifts in multiple contexts without motor planning.J Neurophysiol. 2016 Oct 1;116(4):1956-1985. doi: 10.1152/jn.00605.2015. Epub 2016 Jul 20. J Neurophysiol. 2016. PMID: 27440248 Free PMC article.
-
Fast gaze reorientations by combined movements of the eye, head, trunk and lower extremities.Exp Brain Res. 2015 May;233(5):1639-50. doi: 10.1007/s00221-015-4238-4. Epub 2015 Mar 12. Exp Brain Res. 2015. PMID: 25761968 Free PMC article.
References
-
- Becker W. Saccades. In: Carpenter RHS, editor. Eye Movements. Macmillan; London: 1991. pp. 95–137.
-
- Becker W, Jürgens R. Gaze saccades to visual targets: does head movement change the metrics. In: Berthoz A, Graf W, Vidal PP, editors. The Head-Neck Sensory Motor System. Oxford Univ. Press; New York: 1992. pp. 427–433.
-
- Becker W, King WM, Fuchs AF, Jürgens R, Johanson G, Kornhuber HH. Accuracy of goal-directed saccades and mechanisms of error correction. In: Fuchs AF, Becker W, editors. Progress in Oculomotor Research. Elsevier; New York: 1981. pp. 29–37.
-
- Bergeron A, Guitton D. Fixation neurons in the superior colliculus encode distance between current and desired gaze positions. Nat Neurosci. 2000;3:932–939. - PubMed
-
- Bergeron A, Guitton D. In multiple-step gaze shifts: omnipause (OPNs) and collicular fixation neurons encode gaze position error; OPNs gate saccades. J Neurophysiol. 2002;88:1726–1742. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

