Cholesterol dependence of varicella-zoster virion entry into target cells
- PMID: 17494071
- PMCID: PMC1933378
- DOI: 10.1128/JVI.00486-07
Cholesterol dependence of varicella-zoster virion entry into target cells
Abstract
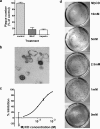
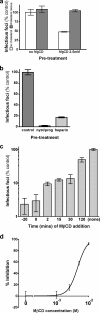
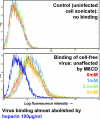
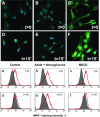
The entry of inhaled virions into airway cells is presumably the initiating step of varicella-zoster infection. In order to characterize viral entry, we studied the relative roles played by lipid rafts and clathrin-mediated transport. Virus and target cells were pretreated with agents designed to perturb selected aspects of endocytosis and membrane composition, and the effects of these perturbations on infectious focus formation were monitored. Infectivity was exquisitely sensitive to methyl-beta-cyclodextrin (M beta CD) and nystatin, which disrupt lipid rafts by removing cholesterol. These agents inhibited infection by enveloped, but not cell-associated, varicella-zoster virus (VZV) in a dose-dependent manner and exerted these effects on both target cell and viral membranes. Inhibition by M beta CD, which could be reversed by cholesterol replenishment, rapidly declined as a function of time after exposure of target cells to VZV, suggesting that an early step in viral infection requires cholesterol. No effect of cholesterol depletion, however, was seen on viral binding; moreover, there was no reduction in the surface expression or internalization of mannose 6-phosphate receptors, which are required for VZV entry. Viral entry was energy dependent and showed concentration-dependent inhibition by chlorpromazine, which, among other actions, blocks clathrin-mediated endocytosis. These data suggest that both membrane lipid composition and clathrin-mediated transport are critical for VZV entry. Lipid rafts are likely to contribute directly to viral envelope integrity and, in the host membrane, may influence endocytosis, evoke downstream signaling, and/or facilitate membrane fusion.
Figures



Similar articles
-
Infection of cells by varicella zoster virus: inhibition of viral entry by mannose 6-phosphate and heparin.Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3546-50. doi: 10.1073/pnas.92.8.3546. Proc Natl Acad Sci U S A. 1995. PMID: 7724595 Free PMC article.
-
Critical role of lipid rafts in virus entry and activation of phosphoinositide 3' kinase/Akt signaling during early stages of Japanese encephalitis virus infection in neural stem/progenitor cells.J Neurochem. 2010 Oct;115(2):537-49. doi: 10.1111/j.1471-4159.2010.06951.x. Epub 2010 Aug 31. J Neurochem. 2010. PMID: 20722967
-
Lipid rafts and HIV pathogenesis: virion-associated cholesterol is required for fusion and infection of susceptible cells.AIDS Res Hum Retroviruses. 2003 Aug;19(8):675-87. doi: 10.1089/088922203322280900. AIDS Res Hum Retroviruses. 2003. PMID: 13678470
-
Insights into the function of tegument proteins from the varicella zoster virus.Sci China Life Sci. 2015 Aug;58(8):739-49. doi: 10.1007/s11427-015-4887-3. Epub 2015 Jul 24. Sci China Life Sci. 2015. PMID: 26208824 Review.
-
Varicella-zoster virus glycoprotein M.Curr Top Microbiol Immunol. 2010;342:147-54. doi: 10.1007/82_2010_30. Curr Top Microbiol Immunol. 2010. PMID: 20373090 Review.
Cited by
-
Quantitative measurement of varicella-zoster virus infection by semiautomated flow cytometry.Appl Environ Microbiol. 2009 Apr;75(7):2027-36. doi: 10.1128/AEM.02006-08. Epub 2009 Feb 5. Appl Environ Microbiol. 2009. PMID: 19201967 Free PMC article.
-
Lipid Rafts: The Maestros of Normal Brain Development.Biomolecules. 2024 Mar 18;14(3):362. doi: 10.3390/biom14030362. Biomolecules. 2024. PMID: 38540780 Free PMC article. Review.
-
The Lord of the NanoRings: Cyclodextrins and the battle against SARS-CoV-2.Int J Pharm. 2020 Oct 15;588:119689. doi: 10.1016/j.ijpharm.2020.119689. Epub 2020 Jul 25. Int J Pharm. 2020. PMID: 32717282 Free PMC article. Review.
-
Role of the lipid rafts in the life cycle of canine coronavirus.J Gen Virol. 2015 Feb;96(Pt 2):331-337. doi: 10.1099/vir.0.070870-0. Epub 2014 Nov 7. J Gen Virol. 2015. PMID: 25381058 Free PMC article.
-
VZV infection of keratinocytes: production of cell-free infectious virions in vivo.Curr Top Microbiol Immunol. 2010;342:173-88. doi: 10.1007/82_2010_13. Curr Top Microbiol Immunol. 2010. PMID: 20225011 Free PMC article. Review.
References
-
- Brown, D. A. 2006. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology 21:430-439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

