Human immunodeficiency virus type 1 replication in dendritic cell-T-cell cocultures is increased upon incorporation of host LFA-1 due to higher levels of virus production in immature dendritic cells
- PMID: 17494076
- PMCID: PMC1933380
- DOI: 10.1128/JVI.02810-06
Human immunodeficiency virus type 1 replication in dendritic cell-T-cell cocultures is increased upon incorporation of host LFA-1 due to higher levels of virus production in immature dendritic cells
Abstract
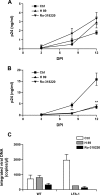
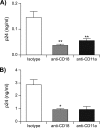
Dendritic cells (DCs) act as a portal for invasion by human immunodeficiency virus type-1 (HIV-1). Here, we investigated whether virion-incorporated host cell membrane proteins can affect virus replication in DC-T-cell cocultures. Using isogenic viruses either devoid of or bearing host-derived leukocyte function-associated antigen 1 (LFA-1), we showed that HIV-1 production is augmented when LFA-1-bearing virions are used compared to that for viral entities lacking this adhesion molecule. This phenomenon was observed in immature monocyte-derived DCs (IM-MDDCs) only and not in DCs displaying a mature phenotype. The increase is not due to higher virus production in responder CD4(+) T cells but rather is linked with a more important productive infection of IM-MDDCs. We provided evidence that virus-associated host LFA-1 molecules do not affect a late event in the HIV-1 life cycle but rather exert an effect on an early step in virus replication. We demonstrated that the enhancement of productive infection of IM-MDDCs that is conferred by virus-anchored host LFA-1 involves the protein kinase A (PKA) and PKC signal transduction pathways. The biological significance of this phenomenon was established by performing experiments with virus stocks produced in primary human cells and anti-LFA-1 antibodies. Together, our results indicate that the association between some virus-bound host proteins and their natural cognate ligands can modulate de novo HIV-1 production by IM-MDDCs. Therefore, the additional interactions between virus-bound host cell membrane constituents and counter receptors on the surfaces of DCs can influence HIV-1 replication in IM-MDDC-T-cell cocultures.
Figures


Similar articles
-
Efficient virus transmission from dendritic cells to CD4+ T cells in response to antigen depends on close contact through adhesion molecules.Virology. 1997 Dec 22;239(2):259-68. doi: 10.1006/viro.1997.8895. Virology. 1997. PMID: 9434717
-
Virion-Associated Vpr Alleviates a Postintegration Block to HIV-1 Infection of Dendritic Cells.J Virol. 2017 Jun 9;91(13):e00051-17. doi: 10.1128/JVI.00051-17. Print 2017 Jul 1. J Virol. 2017. PMID: 28424288 Free PMC article.
-
The importance of virus-associated host ICAM-1 in human immunodeficiency virus type 1 dissemination depends on the cellular context.FASEB J. 2004 Aug;18(11):1294-6. doi: 10.1096/fj.04-1755fje. Epub 2004 Jun 18. FASEB J. 2004. PMID: 15208262
-
Virus infection of dendritic cells: portal for host invasion and host defense.Trends Microbiol. 2004 Jul;12(7):337-45. doi: 10.1016/j.tim.2004.05.003. Trends Microbiol. 2004. PMID: 15223061 Review.
-
Role of cellular adhesion molecules in HIV type 1 infection and their impact on virus neutralization.AIDS Res Hum Retroviruses. 1998 Oct;14 Suppl 3:S247-54. AIDS Res Hum Retroviruses. 1998. PMID: 9814951 Review.
Cited by
-
Efficient replication of human immunodeficiency virus type 1 in resting CD4+ T lymphocytes is induced by coculture with autologous dendritic cells in the absence of foreign antigens.J Virol. 2009 Mar;83(6):2778-82. doi: 10.1128/JVI.01420-08. Epub 2008 Dec 24. J Virol. 2009. PMID: 19109380 Free PMC article.
-
Leishmania infantum amastigotes enhance HIV-1 production in cocultures of human dendritic cells and CD4 T cells by inducing secretion of IL-6 and TNF-alpha.PLoS Negl Trop Dis. 2009 May 26;3(5):e441. doi: 10.1371/journal.pntd.0000441. PLoS Negl Trop Dis. 2009. PMID: 19468304 Free PMC article.
-
Complement opsonization of HIV-1 enhances the uptake by dendritic cells and involves the endocytic lectin and integrin receptor families.PLoS One. 2011;6(8):e23542. doi: 10.1371/journal.pone.0023542. Epub 2011 Aug 11. PLoS One. 2011. PMID: 21853149 Free PMC article.
-
Dendritic cell immunoreceptor is a new target for anti-AIDS drug development: identification of DCIR/HIV-1 inhibitors.PLoS One. 2013 Jul 9;8(7):e67873. doi: 10.1371/journal.pone.0067873. Print 2013. PLoS One. 2013. PMID: 23874461 Free PMC article.
-
Peering into the HIV reservoir.Rev Med Virol. 2018 Jul;28(4):e1981. doi: 10.1002/rmv.1981. Epub 2018 May 9. Rev Med Virol. 2018. PMID: 29744964 Free PMC article. Review.
References
-
- Amiel, C., M. C. Bene, T. May, P. Canton, and G. C. Faure. 1988. LFA1 expression in HIV infection. AIDS 2:211-214. - PubMed
-
- Amyere, M., M. Mettlen, P. Van Der Smissen, A. Platek, B. Payrastre, A. Veithen, and P. J. Courtoy. 2002. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int. J. Med. Microbiol. 291:487-494. - PubMed
-
- Ancuta, P., Y. Bakri, N. Chomont, H. Hocini, D. Gabuzda, and N. Haeffner-Cavaillon. 2001. Opposite effects of IL-10 on the ability of dendritic cells and macrophages to replicate primary CXCR4-dependent HIV-1 strains. J. Immunol. 166:4244-4253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

