Seroprevalence of bactericidal and anti-outer membrane vesicle antibodies to Neisseria meningitidis group B in England
- PMID: 17494636
- PMCID: PMC1951059
- DOI: 10.1128/CVI.00102-07
Seroprevalence of bactericidal and anti-outer membrane vesicle antibodies to Neisseria meningitidis group B in England
Abstract
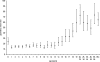
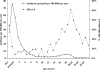
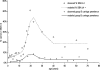
Outer membrane vesicle (OMV) and recombinant protein-based vaccines targeted against multiple strains of group B meningococci are under development. The serum bactericidal antibody (SBA) assay has been designated the surrogate of protection, but the exact cutoff has not been determined. We measured the SBA titers in 2,415 serum samples and the anti-OMV IgG antibody concentrations in 2,672 serum samples representative of the English population to establish a baseline of natural immunity. SBA and anti-OMV IgG antibody titers are high in infants in the first 3 months of life, declining thereafter, presumably as maternal immunity wanes. About 6% of the subjects in the 1- to 11-year-old age group had SBA titers >or=4. During the teenage years, there was a marked increase in the percentage of subjects with SBA titers >or=4, rising to over 50% in 19-year-olds, with about 20% of older adults achieving this titer. The peak in SBA and anti-OMV IgG titers coincided with the peak in meningococcal carriage. Simple mathematical models confirm that the relationship between observed seroprevalence and carriage by age is consistent with carriage inducing SBA and that following an episode of carriage, SBA levels may remain elevated for many months. With the exception of children aged 3 to 11 months, there was no clear relationship between disease incidence and seroprevalence.
Figures
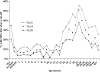

Similar articles
-
Age-specific seroprevalence of serogroup C meningococcal serum bactericidal antibody activity and serogroup A, C, W135 and Y-specific IgG concentrations in the Turkish population during 2005.Vaccine. 2007 Oct 10;25(41):7233-7. doi: 10.1016/j.vaccine.2007.07.019. Epub 2007 Aug 1. Vaccine. 2007. PMID: 17707957
-
Meningococcal surrogates of protection--serum bactericidal antibody activity.Vaccine. 2005 Mar 18;23(17-18):2222-7. doi: 10.1016/j.vaccine.2005.01.051. Vaccine. 2005. PMID: 15755600 Review.
-
Development and characterisation of outer membrane vesicle vaccines against serogroup A Neisseria meningitidis.Vaccine. 2005 May 31;23(29):3762-74. doi: 10.1016/j.vaccine.2005.02.021. Epub 2005 Mar 14. Vaccine. 2005. PMID: 15893613
-
New zealand epidemic strain meningococcal B outer membrane vesicle vaccine in children aged 16-24 months.Pediatr Infect Dis J. 2007 Apr;26(4):345-50. doi: 10.1097/01.inf.0000258697.05341.2c. Pediatr Infect Dis J. 2007. PMID: 17414400 Clinical Trial.
-
Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease.Vaccine. 2009 Jun 24;27 Suppl 2:B112-6. doi: 10.1016/j.vaccine.2009.04.065. Epub 2009 May 21. Vaccine. 2009. PMID: 19464093 Review.
Cited by
-
Commensal Neisseria Kill Neisseria gonorrhoeae through a DNA-Dependent Mechanism.Cell Host Microbe. 2019 Aug 14;26(2):228-239.e8. doi: 10.1016/j.chom.2019.07.003. Epub 2019 Aug 1. Cell Host Microbe. 2019. PMID: 31378677 Free PMC article.
-
Seroprevalence of bactericidal antibodies against serogroup B and C Meningococci in a University Hospital.Braz J Med Biol Res. 2017 Apr 20;50(5):e5590. doi: 10.1590/1414-431X20175590. Braz J Med Biol Res. 2017. PMID: 28443987 Free PMC article.
-
Investigation of correlates of protection against pharyngeal carriage of Neisseria meningitidis genogroups W and Y in the African meningitis belt.PLoS One. 2017 Aug 10;12(8):e0182575. doi: 10.1371/journal.pone.0182575. eCollection 2017. PLoS One. 2017. PMID: 28796795 Free PMC article.
-
Impact of meningococcal group B OMV vaccines, beyond their brief.Hum Vaccin Immunother. 2018 May 4;14(5):1058-1063. doi: 10.1080/21645515.2017.1381810. Epub 2017 Nov 17. Hum Vaccin Immunother. 2018. PMID: 29048985 Free PMC article.
-
Hybrid response to SARS-CoV-2 and Neisseria meningitidis C after an OMV-adjuvanted immunization in mice and their offspring.Hum Vaccin Immunother. 2024 Dec 31;20(1):2346963. doi: 10.1080/21645515.2024.2346963. Epub 2024 May 15. Hum Vaccin Immunother. 2024. PMID: 38745461 Free PMC article.
References
-
- Borrow, R., I. S. Aaberge, G. F. Santos, T. L. Eudey, P. Oster, A. Glennie, J. Findlow, E. A. Hoiby, E. Rosenqvist, P. Balmer, and D. Martin. 2005. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup B, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin. Diagn. Lab. Immunol. 12970-976. - PMC - PubMed
-
- Borrow, R., G. M. Carlone, N. Rosenstein, M. Blake, I. Feavers, D. Martin, W. Zollinger, J. Robbins, I. Aaberge, D. M. Granoff, E. Miller, B. Plikaytis, L. van Alphen, J. Poolman, R. Rappuoli, L. Danzig, J. Hackell, B. Danve, M. Caulfield, S. Lambert, and D. Stephens. 2006. Neisseria meningitidis group B correlates of protection and assay standardization—international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine 245093-5107. - PubMed
-
- Borrow, R., and E. Miller. 2006. Surrogates of protection, p. 323-341. In M. Frosch and M. C. Maiden (ed.), Handbook of meningococcal disease: infection biology, vaccination, clinical management. Wiley-VCH, Weinheim, Germany.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

