Induction of specific immune responses by severe acute respiratory syndrome coronavirus spike DNA vaccine with or without interleukin-2 immunization using different vaccination routes in mice
- PMID: 17494640
- PMCID: PMC1951058
- DOI: 10.1128/CVI.00019-07
Induction of specific immune responses by severe acute respiratory syndrome coronavirus spike DNA vaccine with or without interleukin-2 immunization using different vaccination routes in mice
Abstract
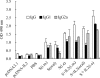
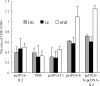
DNA vaccines induce humoral and cellular immune responses in animal models and humans. To analyze the immunogenicity of the severe acute respiratory syndrome (SARS) coronavirus (CoV), SARS-CoV, spike DNA vaccine and the immunoregulatory activity of interleukin-2 (IL-2), DNA vaccine plasmids pcDNA-S and pcDNA-IL-2 were constructed and inoculated into BALB/c mice with or without pcDNA-IL-2 by using three different immunization routes (the intramuscular route, electroporation, or the oral route with live attenuated Salmonella enterica serovar Typhimurium). The cellular and humoral immune responses were assessed by enzyme-linked immunosorbent assays, lymphocyte proliferation assays, enzyme-linked immunospot assays, and fluorescence-activated cell sorter analyses. The results showed that specific humoral and cellular immunities could be induced in mice by inoculating them with SARS-CoV spike DNA vaccine alone or by coinoculation with IL-2-expressing plasmids. In addition, the immune response levels in the coinoculation groups were significantly higher than those in groups receiving the spike DNA vaccine alone. The comparison between the three vaccination routes indicated that oral vaccination evoked a vigorous T-cell response and a weak response predominantly with subclass immunoglobulin G2a (IgG2a) antibody. However, intramuscular immunization evoked a vigorous antibody response and a weak T-cell response, and vaccination by electroporation evoked a vigorous response with a predominant subclass IgG1 antibody response and a moderate T-cell response. Our findings show that the spike DNA vaccine has good immunogenicity and can induce specific humoral and cellular immunities in BALB/c mice, while IL-2 plays an immunoadjuvant role and enhances the humoral and cellular immune responses. Different vaccination routes also evoke distinct immune responses. This study provides basic information for the design of DNA vaccines against SARS-CoV.
Figures



References
-
- Ashby, D., I. Leduc, W. Lauzon, B. C. Lee, N. Sinqhal, and D. W. Cameron. 2005. Attenuated Salmonella typhimurium SL3261 as a vaccine vector for recombinant antigen in rabbits. J. Immunol. Methods 299153-164. - PubMed
-
- Barouch, D. H., A. Craiu, M. J. Kuroda, J. E. Schmitz, X. X. Zheng, S. Santra, J. D. Frost, G. R. Krivulka, M. A. Lifton, C. L. Crabbs, G. Heidecker, H. C. Perry, M. E. Davies, H. Xie, C. E. Nickerson, T. D. Steenbeke, C. I. Lord, D. C. Montefiori, T. B. Strom, J. W. Shiver, M. G. Lewis, and N. L. Letvin. 2000. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. USA 974192-4197. - PMC - PubMed
-
- Barouch, D. H., S. Santra, K. Tenner-Racz, P. Racz, M. J. Kuroda, J. E. Schmitz, S. S. Jackson, M. A. Lifton, D. C. Freed, H. C. Perry, M. E. Davies, J. W. Shiver, and N. L. Letvin. 2002. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J. Immunol. 168562-568. - PubMed
-
- Benyacoub, J., S. Hopkins, A. Potts, S. Kelly, J. P. Kraehenbühl, R. Curtiss, P. De Grandi, and D. N. Haefliger. 1999. The nature of the attenuation of Salmonella typhimurium strains expressing human papillomavirus type 16 virus-like particles determines the systemic and mucosal antibody responses in nasally immunized mice. Infect. Immun. 673674-3679. - PMC - PubMed
-
- Caruso, A., S. Licenziati, M. Corulli, A. D. Canaris, M. A. De Francesco, S. Fiorentini, L. Peroni, F. Fallacara, F. Dima, A. Balsari, and A. Turano. 1997. Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Cytometry 2771-76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

