Rhythmic episodes of subthreshold membrane potential oscillations in the rat inferior olive nuclei in vivo
- PMID: 17494690
- PMCID: PMC6672369
- DOI: 10.1523/JNEUROSCI.5187-06.2007
Rhythmic episodes of subthreshold membrane potential oscillations in the rat inferior olive nuclei in vivo
Abstract
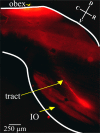
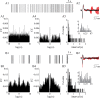
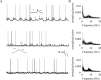
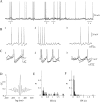
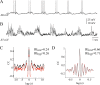
In vitro studies of inferior olive neurons demonstrate that they are intrinsically active, generating periodic spatiotemporal patterns. These self-generated patterns of activity extend the role of olivary neurons beyond that of a deliverer of teaching or error signals. However, autorhythmicity or patterned activity of complex spikes in the cerebellar cortex was observed in only a few studies. This discrepancy between the self-generated rhythmicity in the inferior olive observed in vitro and the sporadic reports on rhythmicity of complex spikes can be reconciled by recording intracellularly from inferior olive neurons in situ. To this end, we recorded intracellularly from olivary neurons of anesthetized rats. We demonstrate that, in vivo, olivary neurons show both slow and fast rhythmic processes. The slow process (0.2-2 Hz) is expressed as rhythmic transitions from quiescent periods to periods of fast rhythm, manifested as subthreshold oscillations of 6-12 Hz. Spikes, if they occur, are locked to the depolarized phase of these subthreshold oscillations and, therefore, hold and transfer rhythmic information. The transient nature of these oscillatory epochs accounts for the difficulties to uncover them by prolonged recordings of complex spikes activity in the cerebellar cortex.
Figures




References
-
- Albus J. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
-
- Chorev E, Manor Y, Yarom Y. Density is destiny—on the relation between quantity of T-Type Ca2+ channels and neuronal electrical behavior. CNS Neurol Disord Drug Targets. 2006;5:655–662. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
