Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo
- PMID: 17494709
- PMCID: PMC6672363
- DOI: 10.1523/JNEUROSCI.5169-06.2007
Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo
Abstract
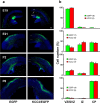
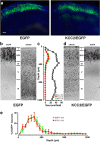
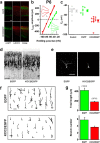
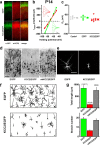
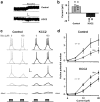
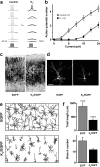
GABA exerts excitatory actions on embryonic and neonatal cortical neurons, but the in vivo function of this GABA excitation is essentially unknown. Using in utero electroporation, we eliminated the excitatory action of GABA in a subpopulation of rat ventricular progenitors and cortical neurons derived from these progenitors by premature expression of the Cl- transporter KCC2, as confirmed by the changes in the reversal potential of GABA-induced currents and the resting membrane potential after GABA(A) receptor blockade. We found that radial migration to layer II/III of the somatosensory cortex of neurons derived from the transfected progenitors was not significantly affected, but their morphological maturation was markedly impaired. Furthermore, reducing neuronal excitability of cortical neurons in vivo by overexpressing an inward-rectifying K+ channel, which lowered the resting membrane potential, mimicked the effect of premature KCC2 expression. Thus, membrane depolarization caused by early GABA excitation is critical for morphological maturation of neonatal cortical neurons in vivo.
Figures

Comment in
-
The influence of GABA on dendritic development in vivo.J Neurosci. 2007 Oct 3;27(40):10649-50. doi: 10.1523/JNEUROSCI.3111-07.2007. J Neurosci. 2007. PMID: 17913898 Free PMC article. Review. No abstract available.
-
GABA excites and sculpts immature neurons well before delivery: modulation by GABA of the development of ventricular progenitor cells.Epilepsy Curr. 2007 Nov-Dec;7(6):167-9. doi: 10.1111/j.1535-7511.2007.00214.x. Epilepsy Curr. 2007. PMID: 18049728 Free PMC article. No abstract available.
Similar articles
-
Developmental up-regulation of KCC2 in the absence of GABAergic and glutamatergic transmission.Eur J Neurosci. 2003 Dec;18(12):3199-206. doi: 10.1111/j.1460-9568.2003.03069.x. Eur J Neurosci. 2003. PMID: 14686894
-
Cortical neurons lacking KCC2 expression show impaired regulation of intracellular chloride.J Neurophysiol. 2005 Mar;93(3):1557-68. doi: 10.1152/jn.00616.2004. Epub 2004 Oct 6. J Neurophysiol. 2005. PMID: 15469961
-
Synaptically-silent immature neurons show gaba and glutamate receptor-mediated currents in adult rat dentate gyrus.Arch Ital Biol. 2006 May;144(2):115-26. Arch Ital Biol. 2006. PMID: 16642790
-
GABA effects during neuronal differentiation of stem cells.Neurochem Res. 2008 Aug;33(8):1546-57. doi: 10.1007/s11064-008-9642-8. Epub 2008 Mar 21. Neurochem Res. 2008. PMID: 18357524 Review.
-
Defining the role of GABA in cortical development.J Physiol. 2009 May 1;587(Pt 9):1873-9. doi: 10.1113/jphysiol.2008.167635. Epub 2009 Jan 19. J Physiol. 2009. PMID: 19153158 Free PMC article. Review.
Cited by
-
Control of cortical neuronal migration by glutamate and GABA.Front Cell Neurosci. 2015 Jan 30;9:4. doi: 10.3389/fncel.2015.00004. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25688185 Free PMC article. Review.
-
NMDA receptors in GABAergic synapses during postnatal development.PLoS One. 2012;7(5):e37753. doi: 10.1371/journal.pone.0037753. Epub 2012 May 25. PLoS One. 2012. PMID: 22662211 Free PMC article.
-
Neural Signaling in Cancer.Annu Rev Neurosci. 2022 Jul 8;45:199-221. doi: 10.1146/annurev-neuro-111020-092702. Epub 2022 Mar 8. Annu Rev Neurosci. 2022. PMID: 35259916 Free PMC article. Review.
-
Interneuron cell types are fit to function.Nature. 2014 Jan 16;505(7483):318-26. doi: 10.1038/nature12983. Nature. 2014. PMID: 24429630 Free PMC article. Review.
-
From Neural Tube Formation Through the Differentiation of Spinal Cord Neurons: Ion Channels in Action During Neural Development.Front Mol Neurosci. 2020 Apr 24;13:62. doi: 10.3389/fnmol.2020.00062. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32390800 Free PMC article.
References
-
- Barbin G, Pollard H, Gaiarsa JL, Ben Ari Y. Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neurosci Lett. 1993;152:150–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
