Differential regulation of endogenous N- and P/Q-type Ca2+ channel inactivation by Ca2+/calmodulin impacts on their ability to support exocytosis in chromaffin cells
- PMID: 17494710
- PMCID: PMC6672387
- DOI: 10.1523/JNEUROSCI.3545-06.2007
Differential regulation of endogenous N- and P/Q-type Ca2+ channel inactivation by Ca2+/calmodulin impacts on their ability to support exocytosis in chromaffin cells
Abstract
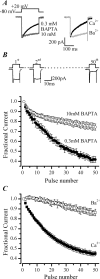
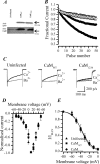
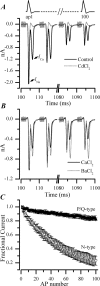
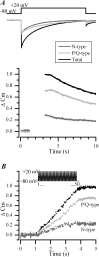
P/Q-type (Ca(V)2.1) and N-type (Ca(V)2.2) Ca2+ channels are critical to stimulus-secretion coupling in the nervous system; feedback regulation of these channels by Ca2+ is therefore predicted to profoundly influence neurotransmission. Here we report divergent regulation of Ca2+-dependent inactivation (CDI) of native N- and P/Q-type Ca2+ channels by calmodulin (CaM) in adult chromaffin cells. Robust CDI of N-type channels was observed in response to prolonged step depolarizations, as well as repetitive stimulation with either brief step depolarizations or action potential-like voltage stimuli. Adenoviral expression of Ca2+-insensitive calmodulin mutants eliminated CDI of N-type channels. This is the first demonstration of CaM-dependent CDI of a native N-type channel. CDI of P/Q-type channels was by comparison modest and insensitive to expression of CaM mutants. Cloning of the C terminus of the Ca(V)2.1 alpha1 subunit from chromaffin cells revealed multiple splice variants lacking structural motifs required for CaM-dependent CDI. The physiological relevance of CDI on stimulus-coupled exocytosis was revealed by combining perforated-patch voltage-clamp recordings of pharmacologically isolated Ca2+ currents with membrane capacitance measurements of exocytosis. Increasing stimulus intensity to invoke CDI resulted in a significant decrease in the exocytotic efficiency of N-type channels compared with P/Q-type channels. Our results reveal unexpected diversity in CaM regulation of native Ca(V)2 channels and suggest that the ability of individual Ca2+ channel subtypes to undergo CDI may be tailored by alternative splicing to meet the specific requirements of a particular cellular function.
Figures




Similar articles
-
R-Type Ca2+ channels are coupled to the rapid component of secretion in mouse adrenal slice chromaffin cells.J Neurosci. 2000 Nov 15;20(22):8323-30. doi: 10.1523/JNEUROSCI.20-22-08323.2000. J Neurosci. 2000. PMID: 11069939 Free PMC article.
-
A perforated patch-clamp study of calcium currents and exocytosis in chromaffin cells of wild-type and alpha(1A) knockout mice.J Neurochem. 2002 Jun;81(5):911-21. doi: 10.1046/j.1471-4159.2002.00845.x. J Neurochem. 2002. PMID: 12065603
-
N- and P/Q-type Ca2+ channels in adrenal chromaffin cells.Acta Physiol (Oxf). 2008 Feb;192(2):247-61. doi: 10.1111/j.1748-1716.2007.01817.x. Epub 2007 Nov 16. Acta Physiol (Oxf). 2008. PMID: 18021320 Review.
-
An activity-dependent increased role for L-type calcium channels in exocytosis is regulated by adrenergic signaling in chromaffin cells.Neuroscience. 2006 Dec 1;143(2):445-59. doi: 10.1016/j.neuroscience.2006.08.001. Epub 2006 Sep 8. Neuroscience. 2006. PMID: 16962713
-
Calcium signaling and exocytosis in adrenal chromaffin cells.Physiol Rev. 2006 Oct;86(4):1093-131. doi: 10.1152/physrev.00039.2005. Physiol Rev. 2006. PMID: 17015485 Review.
Cited by
-
Neuronal calcium sensor-1 regulation of calcium channels, secretion, and neuronal outgrowth.Cell Mol Neurobiol. 2010 Nov;30(8):1283-92. doi: 10.1007/s10571-010-9588-7. Epub 2010 Nov 23. Cell Mol Neurobiol. 2010. PMID: 21104311 Free PMC article. Review.
-
The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential.Pharmacol Rev. 2015 Oct;67(4):821-70. doi: 10.1124/pr.114.009654. Pharmacol Rev. 2015. PMID: 26362469 Free PMC article. Review.
-
"Slow" Voltage-Dependent Inactivation of CaV2.2 Calcium Channels Is Modulated by the PKC Activator Phorbol 12-Myristate 13-Acetate (PMA).PLoS One. 2015 Jul 29;10(7):e0134117. doi: 10.1371/journal.pone.0134117. eCollection 2015. PLoS One. 2015. PMID: 26222492 Free PMC article.
-
Dynamic phospholipid interaction of β2e subunit regulates the gating of voltage-gated Ca2+ channels.J Gen Physiol. 2015 Jun;145(6):529-41. doi: 10.1085/jgp.201411349. Epub 2015 May 11. J Gen Physiol. 2015. PMID: 25964431 Free PMC article.
-
Stabilization of Ca current in Purkinje neurons during high-frequency firing by a balance of Ca-dependent facilitation and inactivation.Channels (Austin). 2009 Nov;3(6):393-401. doi: 10.4161/chan.3.6.9838. Epub 2009 Nov 13. Channels (Austin). 2009. PMID: 19806011 Free PMC article.
References
-
- Ashery U, Betz A, Xu T, Brose N, Rettig J. An efficient method for infection of adrenal chromaffin cells using the Semliki Forest virus gene expression system. Eur J Cell Biol. 1999;78:525–532. - PubMed
-
- Becherer U, Moser T, Stuhmer W, Oheim M. Calcium regulates exocytosis at the level of single vesicles. Nat Neurosci. 2003;6:846–853. - PubMed
-
- Bezprozvanny I, Scheller RH, Tsien RW. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature. 1995;378:623–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
