Comparison of various blood compartments and reporting units for the detection and quantification of Epstein-Barr virus in peripheral blood
- PMID: 17494720
- PMCID: PMC1932972
- DOI: 10.1128/JCM.02308-06
Comparison of various blood compartments and reporting units for the detection and quantification of Epstein-Barr virus in peripheral blood
Abstract
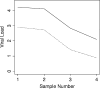
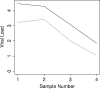
Epstein-Barr virus (EBV) infection is associated with a broad spectrum of disease. While quantification of EBV nucleic acid in the peripheral blood has been demonstrated to be useful for diagnosis and patient care, the optimal sample type and reporting format for such testing remain uncertain. Using quantitative real-time PCR (QRT-PCR), we evaluated EBV in whole blood (WB), peripheral blood mononuclear cells (PBMC), and plasma in 249 samples from 122 patients. In WB and PBMC, results were reported both in viral copies/ml and in copies/microg of total DNA. Trendings of quantitative values over time among the different sample types were compared. The sensitivities of QRT-PCR using WB and that using PBMC did not differ significantly (P = 0.33), and both were more sensitive than plasma alone (P < 0.0001). EBV viral load results from WB and PBMC paired sample types also showed a significant correlation (P < 0.05), as did results reported in copies/ml and copies/microg DNA for both WB and PBMC (R2 > 0.93). EBV viral loads detected using WB and PBMC trended very closely for the few patients who had multiple positive samples available for analysis. WB and PBMC show comparable sensitivities and a close quantitative correlation when assayed for EBV by QRT-PCR. The close correlation between copies/ml and copies/microg DNA also suggests that normalization to cell number or genomic DNA in cellular specimens may not be necessary.
Figures



Similar articles
-
Comparison of six different specimen types for Epstein-Barr viral load quantification in peripheral blood of pediatric patients after heart transplantation or after allogeneic hematopoietic stem cell transplantation.J Clin Virol. 2012 Mar;53(3):186-94. doi: 10.1016/j.jcv.2011.11.010. Epub 2011 Dec 17. J Clin Virol. 2012. PMID: 22182950
-
[Epstein Barr viral load monitoring in mononuclear lymphocytes and serum of renal transplant recipients using a quantitative PCR protocol].G Ital Nefrol. 2003 Mar-Apr;20(2):170-5. G Ital Nefrol. 2003. PMID: 12746803 Italian.
-
Epstein Barr viral load monitoring by quantitative PCR in renal transplant patients.New Microbiol. 2003 Apr;26(2):141-9. New Microbiol. 2003. PMID: 12737195
-
Assessment of automated DNA extraction coupled with real-time PCR for measuring Epstein-Barr virus load in whole blood, peripheral mononuclear cells and plasma.J Clin Virol. 2004 Jun;30(2):157-64. doi: 10.1016/j.jcv.2003.10.002. J Clin Virol. 2004. PMID: 15125872
-
The Biology and Clinical Utility of EBV Monitoring in Blood.Curr Top Microbiol Immunol. 2015;391:475-99. doi: 10.1007/978-3-319-22834-1_17. Curr Top Microbiol Immunol. 2015. PMID: 26428386 Review.
Cited by
-
When kissing (disease) counts.Blood. 2016 Apr 21;127(16):1947-8. doi: 10.1182/blood-2016-01-692087. Blood. 2016. PMID: 27103742 Free PMC article.
-
Epstein-Barr virus: general factors, virus-related diseases and measurement of viral load after transplant.Rev Bras Hematol Hemoter. 2011;33(5):383-8. doi: 10.5581/1516-8484.20110103. Rev Bras Hematol Hemoter. 2011. PMID: 23049344 Free PMC article.
-
Measurement of Epstein-Barr virus DNA load using a novel quantification standard containing two EBV DNA targets and SYBR Green I dye.Virol J. 2010 Sep 22;7:252. doi: 10.1186/1743-422X-7-252. Virol J. 2010. PMID: 20860842 Free PMC article.
-
Hepatitis C virus RNA quantitation in venous and capillary small-volume whole-blood samples.J Clin Microbiol. 2009 Oct;47(10):3231-40. doi: 10.1128/JCM.00925-09. Epub 2009 Aug 19. J Clin Microbiol. 2009. PMID: 19692560 Free PMC article.
-
Post Transplant Lymphoproliferative Disorder.Indian J Hematol Blood Transfus. 2020 Apr;36(2):229-237. doi: 10.1007/s12288-019-01182-x. Epub 2019 Sep 17. Indian J Hematol Blood Transfus. 2020. PMID: 32425371 Free PMC article. Review.
References
-
- Bai, X., et al. 1997. Quantitative polymerase chain reaction for human herpesvirus diagnosis and measurement of Epstein-Barr virus burden in posttransplant lymphoproliferative disorder. Clin. Chem. 43:1843-1849. - PubMed
-
- Gallagher, A., et al. 1999. Detection of Epstein-Barr virus (EBV) genomes in the serum of patients with EBV-associated Hodgkin's disease. Int. J. Cancer 84:442-448. - PubMed
-
- Gan, Y. J., J. L. Sullivan, and J. W. Sixbey. 1994. Detection of cell-free Epstein-Barr virus DNA in serum during acute infectious mononucleosis. J. Infect. Dis. 170:436-439. - PubMed
-
- Green, M., et al. 2000. Predictive negative value of persistent low Epstein-Barr virus viral load after intestinal transplantation in children. Transplantation 70:593-596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

