Hepatitis C virus epitope-specific neutralizing antibodies in Igs prepared from human plasma
- PMID: 17494735
- PMCID: PMC1866310
- DOI: 10.1073/pnas.0703039104
Hepatitis C virus epitope-specific neutralizing antibodies in Igs prepared from human plasma
Abstract
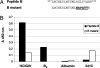
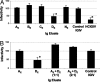
Neutralizing antibodies directed against hepatitis C virus (HCV) are present in Igs made from anti-HCV-positive plasma. However, these HCV-specific Igs are largely ineffective in vivo. The mechanism for the poor effectiveness is currently unknown. We hypothesize that the presence of nonneutralizing antibodies in HCV-specific Igs interferes with the function of neutralizing antibodies, resulting in the reduction or blockage of their effect. In the present study, we identified at least two epitopes at amino acid residues 412-419 (epitope I) and 434-446 (epitope II), located downstream of the hypervariable region I within the HCV E2 protein. We demonstrated that epitope I, but not epitope II, was implicated in HCV neutralization and that binding of a nonneutralizing antibody to epitope II completely disrupted virus neutralization mediated by antibody binding at epitope I. The dynamic interaction between nonneutralizing and neutralizing antibodies may thus play a key role in determining the outcomes of HCV infection. Further exploration of this interplay should lead to a better understanding of the mechanisms of neutralization and immune escape and may indicate pathways for the manufacture of an effective HCV-specific Ig product for immune prophylaxis of HCV infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

