The neoselectionist theory of genome evolution
- PMID: 17494746
- PMCID: PMC1866311
- DOI: 10.1073/pnas.0701652104
The neoselectionist theory of genome evolution
Abstract
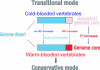
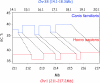
The vertebrate genome is a mosaic of GC-poor and GC-rich isochores, megabase-sized DNA regions of fairly homogeneous base composition that differ in relative amount, gene density, gene expression, replication timing, and recombination frequency. At the emergence of warm-blooded vertebrates, the gene-rich, moderately GC-rich isochores of the cold-blooded ancestors underwent a GC increase. This increase was similar in mammals and birds and was maintained during the evolution of mammalian and avian orders. Neither the GC increase nor its conservation can be accounted for by the random fixation of neutral or nearly neutral single-nucleotide changes (i.e., the vast majority of nucleotide substitutions) or by a biased gene conversion process occurring at random genome locations. Both phenomena can be explained, however, by the neoselectionist theory of genome evolution that is presented here. This theory fully accepts Ohta's nearly neutral view of point mutations but proposes in addition (i) that the AT-biased mutational input present in vertebrates pushes some DNA regions below a certain GC threshold; (ii) that these lower GC levels cause regional changes in chromatin structure that lead to deleterious effects on replication and transcription; and (iii) that the carriers of these changes undergo negative (purifying) selection, the final result being a compositional conservation of the original isochore pattern in the surviving population. Negative selection may also largely explain the GC increase accompanying the emergence of warm-blooded vertebrates. In conclusion, the neoselectionist theory not only provides a solution to the neutralist/selectionist debate but also introduces an epigenomic component in genome evolution.
Conflict of interest statement
The author declares no conflict of interest.
Figures




References
-
- Darwin C. On the Origin of Species, a Facsimile of the First Edition with an Introduction by Ernst Mayr. Cambridge, MA: Harvard Univ Press; 1859. reprinted (1964)
-
- Wallace AR. J Proc Linn Soc (Zoology) 1858;3:53–62.
-
- Fisher RA. The Genetical Theory of Natural Selection. Oxford: Clarendon; 1930.
-
- Haldane JBS. The Causes of Evolution. London: Longmans, Green, and Co; 1932.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

