PI4P promotes the recruitment of the GGA adaptor proteins to the trans-Golgi network and regulates their recognition of the ubiquitin sorting signal
- PMID: 17494868
- PMCID: PMC1924815
- DOI: 10.1091/mbc.e06-10-0897
PI4P promotes the recruitment of the GGA adaptor proteins to the trans-Golgi network and regulates their recognition of the ubiquitin sorting signal
Abstract
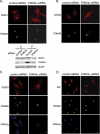
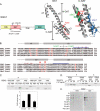
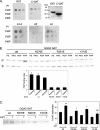
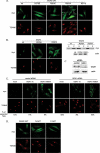
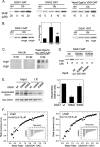
Phosphatidylinositol 4 phosphate (PI4P) is highly enriched in the trans-Golgi network (TGN). Here we establish that PI4P is a key regulator of the recruitment of the GGA clathrin adaptor proteins to the TGN and that PI4P has a novel role in promoting their recognition of the ubiquitin (Ub) sorting signal. Knockdown of PI4KIIalpha by RNA interference (RNAi), which depletes the TGN's PI4P, impaired the recruitment of the GGAs to the TGN. GGAs bind PI4P primarily through their GAT domain, in a region called C-GAT, which also binds Ub but not Arf1. We identified two basic residues in the GAT domain that are essential for PI4P binding in vitro and for the recruitment of GGAs to the TGN in vivo. Unlike wild-type GGA, GGA with mutated GATs failed to rescue the abnormal TGN phenotype of the GGA RNAi-depleted cells. These residues partially overlap with those that bind Ub, and PI4P increased the affinity of the GAT domain for Ub. Because the recruitment of clathrin adaptors and their cargoes to the TGN is mediated through a web of low-affinity interactions, our results show that the dual roles of PI4P can promote specific GGA targeting and cargo recognition at the TGN.
Figures
References
-
- Akutsu M., Kawasaki M., Katoh Y., Shiba T., Yamaguchi Y., Kato R., Kato K., Nakayama K., Wakatsuki S. Structural basis for recognition of ubiquitinated cargo by Tom1-GAT domain. FEBS Lett. 2005;579:5385–5391. - PubMed
-
- Barylko B., Gerber S. H., Binns D. D., Grichine N., Khvotchev M., Sudhof T. C., Albanesi J. P. A novel family of phosphatidylinositol 4-kinases conserved from yeast to humans. J. Biol. Chem. 2001;276:7705–7708. - PubMed
-
- Behnia R., Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. - PubMed
-
- Bonifacino J. S. The GGA proteins: adaptors on the move. Nat. Rev. Mol. Cell Biol. 2004;5:23–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

