Anchoring of protein kinase A-regulatory subunit IIalpha to subapically positioned centrosomes mediates apical bile canalicular lumen development in response to oncostatin M but not cAMP
- PMID: 17494870
- PMCID: PMC1924835
- DOI: 10.1091/mbc.e06-08-0732
Anchoring of protein kinase A-regulatory subunit IIalpha to subapically positioned centrosomes mediates apical bile canalicular lumen development in response to oncostatin M but not cAMP
Abstract
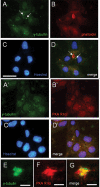
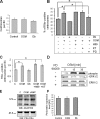
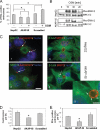
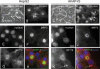
Oncostatin M and cAMP signaling stimulate apical surface-directed membrane trafficking and apical lumen development in hepatocytes, both in a protein kinase A (PKA)-dependent manner. Here, we show that oncostatin M, but not cAMP, promotes the A-kinase anchoring protein (AKAP)-dependent anchoring of the PKA regulatory subunit (R)IIalpha to subapical centrosomes and that this requires extracellular signal-regulated kinase 2 activation. Stable expression of the RII-displacing peptide AKAP-IS, but not a scrambled peptide, inhibits the association of RIIalpha with centrosomal AKAPs and results in the repositioning of the centrosome from a subapical to a perinuclear location. Concomitantly, common endosomes, but not apical recycling endosomes, are repositioned from a subapical to a perinuclear location, without significant effects on constitutive or oncostatin M-stimulated basolateral-to-apical transcytosis. Importantly, however, the expression of the AKAP-IS peptide completely blocks oncostatin M-, but not cAMP-stimulated apical lumen development. Together, the data suggest that centrosomal anchoring of RIIalpha and the interrelated subapical positioning of these centrosomes is required for oncostatin M-, but not cAMP-mediated, bile canalicular lumen development in a manner that is uncoupled from oncostatin M-stimulated apical lumen-directed membrane trafficking. The results also imply that multiple PKA-mediated signaling pathways control apical lumen development and that subapical centrosome positioning is important in some of these pathways.
Figures

Similar articles
-
Regulatory subunit I-controlled protein kinase A activity is required for apical bile canalicular lumen development in hepatocytes.J Biol Chem. 2009 Jul 31;284(31):20773-80. doi: 10.1074/jbc.M109.013599. Epub 2009 May 22. J Biol Chem. 2009. PMID: 19465483 Free PMC article.
-
Efficient trafficking of MDR1/P-glycoprotein to apical canalicular plasma membranes in HepG2 cells requires PKA-RIIalpha anchoring and glucosylceramide.Mol Biol Cell. 2006 Aug;17(8):3638-50. doi: 10.1091/mbc.e06-03-0230. Epub 2006 May 24. Mol Biol Cell. 2006. PMID: 16723498 Free PMC article.
-
Oncostatin M-stimulated apical plasma membrane biogenesis requires p27(Kip1)-regulated cell cycle dynamics.Mol Biol Cell. 2004 Sep;15(9):4105-14. doi: 10.1091/mbc.e04-03-0201. Epub 2004 Jul 7. Mol Biol Cell. 2004. PMID: 15240818 Free PMC article.
-
Mechanisms of protein kinase A anchoring.Int Rev Cell Mol Biol. 2010;283:235-330. doi: 10.1016/S1937-6448(10)83005-9. Int Rev Cell Mol Biol. 2010. PMID: 20801421 Review.
-
The biological functions of A-kinase anchor proteins.J Mol Biol. 2001 Apr 27;308(2):99-114. doi: 10.1006/jmbi.2001.4585. J Mol Biol. 2001. PMID: 11327755 Review.
Cited by
-
The Na+/H+ exchanger NHE6 in the endosomal recycling system is involved in the development of apical bile canalicular surface domains in HepG2 cells.Mol Biol Cell. 2010 Apr 1;21(7):1293-304. doi: 10.1091/mbc.e09-09-0767. Epub 2010 Feb 3. Mol Biol Cell. 2010. PMID: 20130086 Free PMC article.
-
Protein kinase A-dependent step(s) in hepatitis C virus entry and infectivity.J Virol. 2008 Sep;82(17):8797-811. doi: 10.1128/JVI.00592-08. Epub 2008 Jun 25. J Virol. 2008. PMID: 18579596 Free PMC article.
-
AKAP350 Is involved in the development of apical "canalicular" structures in hepatic cells HepG2.J Cell Physiol. 2012 Jan;227(1):160-71. doi: 10.1002/jcp.22713. J Cell Physiol. 2012. PMID: 21374596 Free PMC article.
-
Apical trafficking in epithelial cells: signals, clusters and motors.J Cell Sci. 2009 Dec 1;122(Pt 23):4253-66. doi: 10.1242/jcs.032615. J Cell Sci. 2009. PMID: 19923269 Free PMC article. Review.
-
Regulatory subunit I-controlled protein kinase A activity is required for apical bile canalicular lumen development in hepatocytes.J Biol Chem. 2009 Jul 31;284(31):20773-80. doi: 10.1074/jbc.M109.013599. Epub 2009 May 22. J Biol Chem. 2009. PMID: 19465483 Free PMC article.
References
-
- Brown P. S., Wang E., Aroeti B., Chapin S. J., Mostov K. E., Dunn K. W. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK). cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. - PubMed
-
- Carlson C. R., Witczak O., Vossebein L., Labbé J. C., Skálhegg B. S., Keryer G., Herberg F. W., Collas P., Taskén K. CDK1-mediated phosphorylation of the RIIα regulatory subunit of PKA works as a molecular switch that promotes dissociation of RIIα from centrosomes at mitosis. J. Cell Sci. 2001;114:3243–3254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

