Recycling endosomes of polarized epithelial cells actively sort apical and basolateral cargos into separate subdomains
- PMID: 17494872
- PMCID: PMC1924834
- DOI: 10.1091/mbc.e05-09-0873
Recycling endosomes of polarized epithelial cells actively sort apical and basolateral cargos into separate subdomains
Abstract
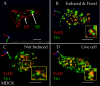
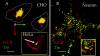
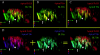
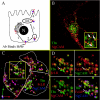
The plasma membranes of epithelial cells plasma membranes contain distinct apical and basolateral domains that are critical for their polarized functions. However, both domains are continuously internalized, with proteins and lipids from each intermixing in supranuclear recycling endosomes (REs). To maintain polarity, REs must faithfully recycle membrane proteins back to the correct plasma membrane domains. We examined sorting within REs and found that apical and basolateral proteins were laterally segregated into subdomains of individual REs. Subdomains were absent in unpolarized cells and developed along with polarization. Subdomains were formed by an active sorting process within REs, which precedes the formation of AP-1B-dependent basolateral transport vesicles. Both the formation of subdomains and the fidelity of basolateral trafficking were dependent on PI3 kinase activity. This suggests that subdomain and transport vesicle formation occur as separate sorting steps and that both processes may contribute to sorting fidelity.
Figures

Similar articles
-
Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome.Mol Biol Cell. 2008 May;19(5):2059-68. doi: 10.1091/mbc.e07-09-0902. Epub 2008 Feb 20. Mol Biol Cell. 2008. PMID: 18287531 Free PMC article.
-
Analyzing the role of AP-1B in polarized sorting from recycling endosomes in epithelial cells.Methods Cell Biol. 2015;130:289-305. doi: 10.1016/bs.mcb.2015.03.023. Epub 2015 Jun 11. Methods Cell Biol. 2015. PMID: 26360041
-
Phosphatidylinositol 3,4,5-trisphosphate localization in recycling endosomes is necessary for AP-1B-dependent sorting in polarized epithelial cells.Mol Biol Cell. 2010 Jan 1;21(1):95-105. doi: 10.1091/mbc.e09-01-0036. Epub 2009 Oct 28. Mol Biol Cell. 2010. PMID: 19864464 Free PMC article.
-
Recycling endosomes in apical plasma membrane domain formation and epithelial cell polarity.Trends Cell Biol. 2010 Oct;20(10):618-26. doi: 10.1016/j.tcb.2010.08.004. Trends Cell Biol. 2010. PMID: 20833047 Review.
-
Role of the epithelial cell-specific clathrin adaptor complex AP-1B in cell polarity.Cell Logist. 2015 Jul 30;5(2):e1074331. doi: 10.1080/21592799.2015.1074331. eCollection 2015 Apr-Jun. Cell Logist. 2015. PMID: 27057418 Free PMC article. Review.
Cited by
-
Sphingomyelin metabolism is involved in the differentiation of MDCK cells induced by environmental hypertonicity.J Lipid Res. 2015 Apr;56(4):786-800. doi: 10.1194/jlr.M050781. Epub 2015 Feb 10. J Lipid Res. 2015. PMID: 25670801 Free PMC article.
-
Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome.Mol Biol Cell. 2008 May;19(5):2059-68. doi: 10.1091/mbc.e07-09-0902. Epub 2008 Feb 20. Mol Biol Cell. 2008. PMID: 18287531 Free PMC article.
-
Clathrin and AP1B: key roles in basolateral trafficking through trans-endosomal routes.FEBS Lett. 2009 Dec 3;583(23):3784-95. doi: 10.1016/j.febslet.2009.10.050. Epub 2009 Oct 23. FEBS Lett. 2009. PMID: 19854182 Free PMC article. Review.
-
The Endo-Lysosomal System of Brain Endothelial Cells Is Influenced by Astrocytes In Vitro.Mol Neurobiol. 2018 Nov;55(11):8522-8537. doi: 10.1007/s12035-018-0988-x. Epub 2018 Mar 20. Mol Neurobiol. 2018. PMID: 29560581
-
Retromer maintains basolateral distribution of the type II TGF-β receptor via the recycling endosome.Mol Biol Cell. 2013 Jul;24(14):2285-98. doi: 10.1091/mbc.E13-02-0093. Epub 2013 May 29. Mol Biol Cell. 2013. PMID: 23720763 Free PMC article.
References
-
- Bomsel M., Parton R., Kuznetsov S. A., Schroer T. A., Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

