The tumor suppressor CYLD regulates entry into mitosis
- PMID: 17495026
- PMCID: PMC1867381
- DOI: 10.1073/pnas.0703268104
The tumor suppressor CYLD regulates entry into mitosis
Abstract
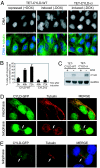
Mutations in the cylindromatosis (CYLD) gene cause benign tumors of skin appendages, referred to as cylindromas. The CYLD gene encodes a deubiquitinating enzyme that removes Lys-63-linked ubiquitin chains from I kappa B kinase signaling components and thereby inhibits NF-kappaB pathway activation. The dysregulation of NF-kappaB activity has been proposed to promote cell transformation in part by increasing apoptosis resistance, but it is not clear whether this is CYLD's only or predominant tumor-suppressing function. Here, we show that CYLD is also required for timely entry into mitosis. Consistent with a cell-cycle regulatory function, CYLD localizes to microtubules in interphase and the midbody during telophase, and its protein levels decrease as cells exit from mitosis. We identified the protein kinase Plk1 as a potential target of CYLD in the regulation of mitotic entry, based on their physical interaction and similar loss-of-function and overexpression phenotypes. Our findings raise the possibility that, as with other genes regulating tumorigenesis, CYLD has not only tumor-suppressing (apoptosis regulation) but also tumor-promoting activities (enhancer of mitotic entry). We propose that this additional function of CYLD could provide an explanation for the benign nature of most cylindroma lesions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, et al. Nat Genet. 2000;25:160–165. - PubMed
-
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Nature. 2003;424:797–801. - PubMed
-
- Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. Nature. 2003;424:801–805. - PubMed
-
- Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. Nature. 2003;424:793–796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

