Contribution of itraconazole metabolites to inhibition of CYP3A4 in vivo
- PMID: 17495874
- PMCID: PMC3488349
- DOI: 10.1038/sj.clpt.6100230
Contribution of itraconazole metabolites to inhibition of CYP3A4 in vivo
Abstract
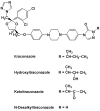
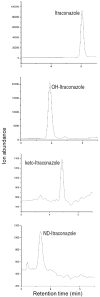
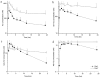
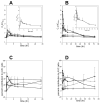
Itraconazole (ITZ) is metabolized in vitro to three inhibitory metabolites: hydroxy-itraconazole (OH-ITZ), keto-itraconazole (keto-ITZ), and N-desalkyl-itraconazole (ND-ITZ). The goal of this study was to determine the contribution of these metabolites to drug-drug interactions caused by ITZ. Six healthy volunteers received 100 mg ITZ orally for 7 days, and pharmacokinetic analysis was conducted at days 1 and 7 of the study. The extent of CYP3A4 inhibition by ITZ and its metabolites was predicted using this data. ITZ, OH-ITZ, keto-ITZ, and ND-ITZ were detected in plasma samples of all volunteers. A 3.9-fold decrease in the hepatic intrinsic clearance of a CYP3A4 substrate was predicted using the average unbound steady-state concentrations (C(ss,ave,u)) and liver microsomal inhibition constants for ITZ, OH-ITZ, keto-ITZ, and ND-ITZ. Accounting for circulating metabolites of ITZ significantly improved the in vitro to in vivo extrapolation of CYP3A4 inhibition compared to a consideration of ITZ exposure alone.
Figures
Similar articles
-
Role of itraconazole metabolites in CYP3A4 inhibition.Drug Metab Dispos. 2004 Oct;32(10):1121-31. doi: 10.1124/dmd.104.000315. Epub 2004 Jul 8. Drug Metab Dispos. 2004. PMID: 15242978
-
Stereochemical aspects of itraconazole metabolism in vitro and in vivo.Drug Metab Dispos. 2006 Apr;34(4):583-90. doi: 10.1124/dmd.105.008508. Epub 2006 Jan 13. Drug Metab Dispos. 2006. PMID: 16415110 Clinical Trial.
-
Dynamically simulating the interaction of midazolam and the CYP3A4 inhibitor itraconazole using individual coupled whole-body physiologically-based pharmacokinetic (WB-PBPK) models.Theor Biol Med Model. 2007 Mar 26;4:13. doi: 10.1186/1742-4682-4-13. Theor Biol Med Model. 2007. PMID: 17386084 Free PMC article.
-
Optimisation of itraconazole therapy using target drug concentrations.Clin Pharmacokinet. 1998 Dec;35(6):461-73. doi: 10.2165/00003088-199835060-00004. Clin Pharmacokinet. 1998. PMID: 9884817 Review.
-
Itraconazole oral solution and intravenous formulations: a review of pharmacokinetics and pharmacodynamics.J Clin Pharm Ther. 2001 Jun;26(3):159-69. doi: 10.1046/j.1365-2710.2001.00338.x. J Clin Pharm Ther. 2001. PMID: 11422598 Review.
Cited by
-
Warfarin-amiodarone drug-drug interactions: determination of [I](u)/K(I,u) for amiodarone and its plasma metabolites.Clin Pharmacol Ther. 2012 Apr;91(4):709-17. doi: 10.1038/clpt.2011.283. Epub 2012 Mar 7. Clin Pharmacol Ther. 2012. PMID: 22398967 Free PMC article.
-
Evaluation of the Potential for Drug-Drug Interactions with Inhaled Itraconazole Using Physiologically Based Pharmacokinetic Modelling, Based on Phase 1 Clinical Data.AAPS J. 2023 Jun 21;25(4):62. doi: 10.1208/s12248-023-00828-z. AAPS J. 2023. PMID: 37344751 Clinical Trial.
-
Ritonavir is the best alternative to ketoconazole as an index inhibitor of cytochrome P450-3A in drug-drug interaction studies.Br J Clin Pharmacol. 2015 Sep;80(3):342-50. doi: 10.1111/bcp.12668. Epub 2015 Jun 1. Br J Clin Pharmacol. 2015. PMID: 25923589 Free PMC article. Review.
-
CYP2C8 but not CYP3A4 is important in the pharmacokinetics of montelukast.Br J Clin Pharmacol. 2012 Feb;73(2):257-67. doi: 10.1111/j.1365-2125.2011.04086.x. Br J Clin Pharmacol. 2012. PMID: 21838784 Free PMC article. Clinical Trial.
-
Multienzyme Kinetics and Sequential Metabolism.Methods Mol Biol. 2021;2342:89-112. doi: 10.1007/978-1-0716-1554-6_4. Methods Mol Biol. 2021. PMID: 34272692
References
-
- Haria M, Bryson HM, Goa KL. Itraconazole. A reappraisal of its pharmacological properties and therapeutic use in the management of superficial fungal infections. Drugs. 1996;51:585–620. - PubMed
-
- Poirier JM, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet. 1998;35:461–473. - PubMed
-
- Schäfer-Körting M. Pharmacokinetic optimisation of oral antifungal therapy. Clin Pharmacokinet. 1993;25:329–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

