Contribution of itraconazole metabolites to inhibition of CYP3A4 in vivo
- PMID: 17495874
- PMCID: PMC3488349
- DOI: 10.1038/sj.clpt.6100230
Contribution of itraconazole metabolites to inhibition of CYP3A4 in vivo
Abstract
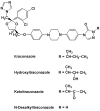
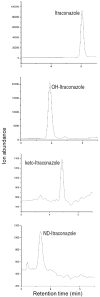
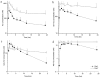
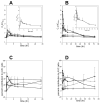
Itraconazole (ITZ) is metabolized in vitro to three inhibitory metabolites: hydroxy-itraconazole (OH-ITZ), keto-itraconazole (keto-ITZ), and N-desalkyl-itraconazole (ND-ITZ). The goal of this study was to determine the contribution of these metabolites to drug-drug interactions caused by ITZ. Six healthy volunteers received 100 mg ITZ orally for 7 days, and pharmacokinetic analysis was conducted at days 1 and 7 of the study. The extent of CYP3A4 inhibition by ITZ and its metabolites was predicted using this data. ITZ, OH-ITZ, keto-ITZ, and ND-ITZ were detected in plasma samples of all volunteers. A 3.9-fold decrease in the hepatic intrinsic clearance of a CYP3A4 substrate was predicted using the average unbound steady-state concentrations (C(ss,ave,u)) and liver microsomal inhibition constants for ITZ, OH-ITZ, keto-ITZ, and ND-ITZ. Accounting for circulating metabolites of ITZ significantly improved the in vitro to in vivo extrapolation of CYP3A4 inhibition compared to a consideration of ITZ exposure alone.
Figures
References
-
- Haria M, Bryson HM, Goa KL. Itraconazole. A reappraisal of its pharmacological properties and therapeutic use in the management of superficial fungal infections. Drugs. 1996;51:585–620. - PubMed
-
- Poirier JM, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet. 1998;35:461–473. - PubMed
-
- Schäfer-Körting M. Pharmacokinetic optimisation of oral antifungal therapy. Clin Pharmacokinet. 1993;25:329–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

