Contrasting patterns in crop domestication and domestication rates: recent archaeobotanical insights from the Old World
- PMID: 17495986
- PMCID: PMC2759199
- DOI: 10.1093/aob/mcm048
Contrasting patterns in crop domestication and domestication rates: recent archaeobotanical insights from the Old World
Abstract
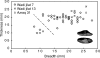
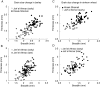
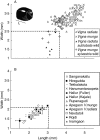
Background: Archaeobotany, the study of plant remains from sites of ancient human activity, provides data for studying the initial evolution of domesticated plants. An important background to this is defining the domestication syndrome, those traits by which domesticated plants differ from wild relatives. These traits include features that have been selected under the conditions of cultivation. From archaeological remains the easiest traits to study are seed size and in cereal crops the loss of natural seed dispersal.
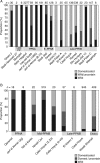
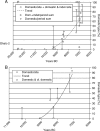
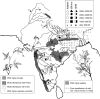
Scope: The rate at which these features evolved and the ordering in which they evolved can now be documented for a few crops of Asia and Africa. This paper explores this in einkorn wheat (Triticum monococcum) and barley (Hordeum vulgare) from the Near East, rice (Oryza sativa) from China, mung (Vigna radiata) and urd (Vigna mungo) beans from India, and pearl millet (Pennisetum glaucum) from west Africa. Brief reference is made to similar data on lentils (Lens culinaris), peas (Pisum sativum), soybean (Glycine max) and adzuki bean (Vigna angularis). Available quantitative data from archaeological finds are compiled to explore changes with domestication. The disjunction in cereals between seed size increase and dispersal is explored, and rates at which these features evolved are estimated from archaeobotanical data. Contrasts between crops, especially between cereals and pulses, are examined.
Conclusions: These data suggest that in domesticated grasses, changes in grain size and shape evolved prior to non-shattering ears or panicles. Initial grain size increases may have evolved during the first centuries of cultivation, within perhaps 500-1000 years. Non-shattering infructescences were much slower, becoming fixed about 1000-2000 years later. This suggests a need to reconsider the role of sickle harvesting in domestication. Pulses, by contrast, do not show evidence for seed size increase in relation to the earliest cultivation, and seed size increase may be delayed by 2000-4000 years. This implies that conditions that were sufficient to select for larger seed size in Poaceae were not sufficient in Fabaceae. It is proposed that animal-drawn ploughs (or ards) provided the selection pressure for larger seeds in legumes. This implies different thresholds of selective pressure, for example in relation to differing seed ontogenetics and underlying genetic architecture in these families. Pearl millet (Pennisetum glaucum) may show some similarities to the pulses in terms of a lag-time before truly larger-grained forms evolved.
Figures





References
-
- Allaby RG, Banerjee M, Brown TA. Evolution of the high molecular weight glutenin loci of the A, B, D, and G genomes of wheat. Genome. 1999;42:116–128. - PubMed
-
- Allard RW. Genetic changes associated with the evolution of adaptedness in cultivated plants and their wild progenitors. The Journal of Heredity. 1988;79:225–238. - PubMed
-
- Allchin FR, Allchin B. Origins of a civilization. The prehistory and early archaeology of South Asia. New Delhi: Penguin Books India; 1997.
-
- Allen H. The Bagundji of the Darling Basin: cereal gatherers in an uncertain environment. World Archaeology. 1974;5:309–322.
-
- Amblard S, Pernes J. The identification of cultivated pearl millet (Pennisetum) amongst plant impressions on pottery from Oued Chebbi (Dhar Oualata, Mauritania) African Archaeological Review. 1989;7:117–126.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

