Membrane insertion and bilayer perturbation by antimicrobial peptide CM15
- PMID: 17496013
- PMCID: PMC1948049
- DOI: 10.1529/biophysj.107.104034
Membrane insertion and bilayer perturbation by antimicrobial peptide CM15
Abstract
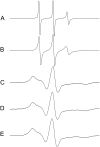
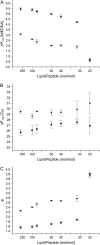
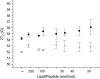
Antimicrobial peptides (AMPs) are an important component of innate immunity and have generated considerable interest as a potential new class of antibiotic. The biological activity of AMPs is strongly influenced by peptide-membrane interactions; however, for many of these peptides the molecular details of how they disrupt and/or translocate across target membranes are not known. CM15 is a linear, synthetic hybrid AMP composed of the first seven residues of the cecropin A and residues 2-9 of the bee venom peptide mellitin. Previous studies have shown that upon membrane binding CM15 folds into an alpha-helix with its helical axis aligned parallel to the bilayer surface and have implicated the formation of 2.2-3.8 nm pores in its bactericidal activity. Here we report site-directed spin labeling electron paramagnetic resonance studies examining the behavior of CM15 analogs labeled with a methanethiosulfonate spin label (MTSL) and a brominated MTSL as a function of increasing peptide concentration and utilize phospholipid-analog spin labels to assess the effects of CM15 binding and accumulation on the physical properties of membrane lipids. We find that as the concentration of membrane-bound CM15 is increased the N-terminal domain of the peptide becomes more deeply immersed in the lipid bilayer. Only minimal changes are observed in the rotational dynamics of membrane lipids, and changes in lipid dynamics are confined primarily to near the membrane surface. However, the accumulation of membrane-bound CM15 dramatically increases accessibility of lipid-analog spin labels to the polar relaxation agent, nickel (II) ethylenediaminediacetate, suggesting an increased permeability of the membrane to polar solutes. These results are discussed in relation to the molecular mechanism of membrane disruption by CM15.
Figures




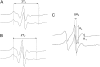

Similar articles
-
Membrane binding, structure, and localization of cecropin-mellitin hybrid peptides: a site-directed spin-labeling study.Biophys J. 2004 Jan;86(1 Pt 1):329-36. doi: 10.1016/S0006-3495(04)74108-9. Biophys J. 2004. PMID: 14695274 Free PMC article.
-
Mechanism of antibacterial action of dermaseptin B2: interplay between helix-hinge-helix structure and membrane curvature strain.Biochemistry. 2009 Jan 20;48(2):313-27. doi: 10.1021/bi802025a. Biochemistry. 2009. PMID: 19113844
-
Using infrared spectroscopy of cyanylated cysteine to map the membrane binding structure and orientation of the hybrid antimicrobial peptide CM15.Biochemistry. 2011 Dec 27;50(51):11097-108. doi: 10.1021/bi200903p. Epub 2011 Dec 2. Biochemistry. 2011. PMID: 22103476 Free PMC article.
-
Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides.Biochim Biophys Acta. 1999 Dec 15;1462(1-2):55-70. doi: 10.1016/s0005-2736(99)00200-x. Biochim Biophys Acta. 1999. PMID: 10590302 Review.
-
AMPs and OMPs: Is the folding and bilayer insertion of β-stranded outer membrane proteins governed by the same biophysical principles as for α-helical antimicrobial peptides?Biochim Biophys Acta. 2015 Sep;1848(9):1944-54. doi: 10.1016/j.bbamem.2015.02.019. Epub 2015 Feb 27. Biochim Biophys Acta. 2015. PMID: 25726906 Review.
Cited by
-
Site-directed spin label EPR studies of the structure and membrane interactions of the bacterial phospholipase ExoU.Appl Magn Reson. 2024 Mar;55(1-3):279-295. doi: 10.1007/s00723-023-01620-0. Epub 2023 Oct 4. Appl Magn Reson. 2024. PMID: 39175603 Free PMC article.
-
Decoupling the Functional Roles of Cationic and Hydrophobic Groups in the Antimicrobial and Hemolytic Activities of Methacrylate Random Copolymers.Biomacromolecules. 2018 Nov 12;19(11):4370-4378. doi: 10.1021/acs.biomac.8b01256. Epub 2018 Oct 26. Biomacromolecules. 2018. PMID: 30350596 Free PMC article.
-
Lysine-enriched cecropin-mellitin antimicrobial peptides with enhanced selectivity.Antimicrob Agents Chemother. 2008 Dec;52(12):4463-5. doi: 10.1128/AAC.00810-08. Epub 2008 Oct 13. Antimicrob Agents Chemother. 2008. PMID: 18852279 Free PMC article.
-
Removal and identification of external protein corona members from RBC-derived extracellular vesicles by surface manipulating antimicrobial peptides.J Extracell Biol. 2023 Mar 8;2(3):e78. doi: 10.1002/jex2.78. eCollection 2023 Mar. J Extracell Biol. 2023. PMID: 38938416 Free PMC article.
-
Transcriptome analysis of Corvus splendens reveals a repertoire of antimicrobial peptides.Sci Rep. 2023 Oct 31;13(1):18728. doi: 10.1038/s41598-023-45875-w. Sci Rep. 2023. PMID: 37907616 Free PMC article.
References
-
- Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61–92. - PubMed
-
- Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature. 415:389–395. - PubMed
-
- Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

