Stability and robustness of an organelle number control system: modeling and measuring homeostatic regulation of centriole abundance
- PMID: 17496020
- PMCID: PMC1948063
- DOI: 10.1529/biophysj.107.107052
Stability and robustness of an organelle number control system: modeling and measuring homeostatic regulation of centriole abundance
Abstract
Control of organelle abundance is a fundamental unsolved problem in cell biology. Mechanisms for number control have been proposed in which organelle assembly is actively increased or decreased to compensate for deviations from a set-point, but such phenomena have not been experimentally verified. In this report we examine the control of centriole copy number. We develop a simple scheme to represent organelle inheritance as a first-order Markov process and describe two figures of merit based on entropy and convergence times that can be used to evaluate performance of organelle number control systems. Using this approach we show that segregation of centrioles by the mitotic spindle can shape the specificity of the steady-state centriole number distribution but is neither necessary nor sufficient for stable restoration of centriole number following perturbations. We then present experimental evidence that living cells can restore correct centriole copy number following transient perturbation, revealing a homeostatic control system. We present evidence that correction occurs at the level of single cell divisions, does not require association of centrioles with the mitotic spindle, and involves modulation of centriole assembly as a function of centriole number during S-phase. Combining our experimental and modeling results, we identify two processes required for error correction, de novo assembly and number-limiting, and show that both processes contribute to robust and stable homeostatic control of centriole number, yielding a system capable of suppressing biological noise at the level of organelle abundance.
Figures

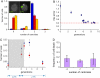
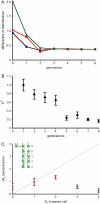

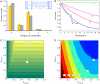
References
-
- Stelling, J., U. Sauer, Z. Szallasi, F. J. Doyle, and J. Doyle. 2004. Robustness of cellular functions. Cell. 118:675–685. - PubMed
-
- El-Samad, H., J. P. Goff, and M. Khammash. 2002. Calcium homeostasis and parturient hypocalcaemia: an integral feedback perspective. J. Theor. Biol. 214:17–29. - PubMed
-
- Tyson, J. J., K. C. Chen, and B. Novak. 2003. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr. Opin. Cell Biol. 15:221–231. - PubMed
-
- Hennis, A. S., and W. Birky. 1984. Stochastic partitioning of chloroplasts at cell division in the alga Olisthodiscus, and compensating control of chloroplast replication. J. Cell Sci. 70:1–15. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

