Gold-nanoparticle-assisted laser perturbation of chromatin assembly reveals unusual aspects of nuclear architecture within living cells
- PMID: 17496030
- PMCID: PMC1959558
- DOI: 10.1529/biophysj.106.102202
Gold-nanoparticle-assisted laser perturbation of chromatin assembly reveals unusual aspects of nuclear architecture within living cells
Abstract
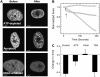
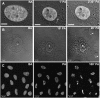
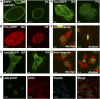
Chromatin organization within the nucleus is a vital regulator of genome function, yet its mechanical coupling to the nuclear architecture has remained elusive. To directly investigate this coupling, we locally modulated chromatin structure in living cells using nanoparticle-based laser perturbation. Unusual differences in the response of the cell nucleus were observed depending on the nuclear region that was perturbed--the heterochromatin, the euchromatin, and the nuclear envelope. This response varied under different conditions of cellular perturbations such as ATP depletion, apoptosis, and inhibition of histone deacetylases. Our studies implicate heterochromatin organization in imparting mechanical stability to the cell nucleus and suggest that nuclear size and shape are the result of interplay between nuclear and cytoplasmic anchors.
Figures



References
-
- Cremer, T., and C. Cremer. 2001. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat. Rev. Genet. 2:292–301. - PubMed
-
- Foster, H. A., and J. M. Bridger. 2005. The genome and the nucleus: a marriage made by evolution. Genome organisation and nuclear architecture. Chromosoma. 114:212–229. - PubMed
-
- Horowitz-Scherer, R. A., and C. L. Woodcock. 2006. Organization of interphase chromatin. Chromosoma. 115:1–14. - PubMed
-
- Kosak, S. T., and M. Groudine. 2004. Gene order and dynamic domains. Science. 306:644–647. - PubMed
-
- Gerlich, D., J. Beaudouin, B. Kalbfuss, N. Daigle, R. Eils, and J. Ellenberg. 2003. Global chromosome positions are transmitted through mitosis in mammalian cells. Cell. 112:751–764. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

