Sodium channels: ionic model of slow inactivation and state-dependent drug binding
- PMID: 17496040
- PMCID: PMC1948041
- DOI: 10.1529/biophysj.106.100248
Sodium channels: ionic model of slow inactivation and state-dependent drug binding
Abstract
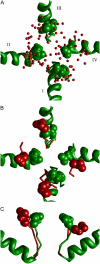
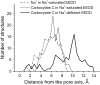
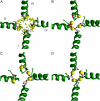
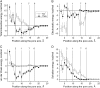
Inactivation is a fundamental property of voltage-gated ion channels. Fast inactivation of Na(+) channels involves channel block by the III-IV cytoplasmic interdomain linker. The mechanisms of nonfast types of inactivation (intermediate, slow, and ultraslow) are unclear, although the ionic environment and P-loops rearrangement appear to be involved. In this study, we employed a TTX-based P-loop domain model of a sodium channel and the MCM method to investigate a possible role of P-loop rearrangement in the nonfast inactivation. Our modeling predicts that Na(+) ions can bind between neighboring domains in the outer-carboxylates ring EEDD, forming an ordered structure with interdomain contacts that stabilize the conducting conformation of the outer pore. In this model, the permeant ions can transit between the EEDD ring and the selectivity filter ring DEKA, retaining contacts with at least two carboxylates. In the absence of Na(+), the electrostatic repulsion between the EEDD carboxylates disrupts the permeable configuration. In this Na(+)-deficient model, the region between the EEDD and DEKA rings is inaccessible for Na(+) but is accessible for TMA. Taken together, these results suggest that Na(+)-saturated models are consistent with experimental characteristics of the open channels, whereas Na(+)-deficient models are consistent with experimentally defined properties of the slow-inactivated channels. Our calculations further predict that binding of LAs to the inner pore would depend on whether Na(+) occupies the DEKA ring. In the absence of Na(+) in the DEKA ring, the cationic group of lidocaine occurs in the focus of the pore helices' macrodipoles and would prevent occupation of the ring by Na(+). Loading the DEKA ring with Na(+) results in the electrostatic repulsion with lidocaine. Thus, there are antagonistic relations between a cationic ligand bound in the inner pore and Na(+) in the DEKA ring.
Figures





References
-
- Ulbricht, W. 2005. Sodium channel inactivation: molecular determinants and modulation. Physiol. Rev. 85:1271–1301. - PubMed
-
- Hoshi, T., W. N. Zagotta, and R. W. Aldrich. 1990. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 250:506–507. - PubMed
-
- Vassilev, P. M., T. Scheuer, and W. A. Catterall. 1988. Identification of an intracellular peptide segment involved in sodium channel inactivation. Science. 241:1658–1661. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

