Lipid lateral segregation driven by diacyl cyclodextrin interactions at the membrane surface
- PMID: 17496041
- PMCID: PMC1948046
- DOI: 10.1529/biophysj.106.099945
Lipid lateral segregation driven by diacyl cyclodextrin interactions at the membrane surface
Erratum in
- Biophys J. 2008 Jan 15;94(2):715
Abstract
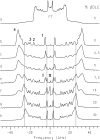
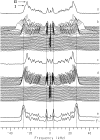
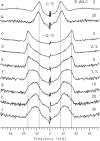
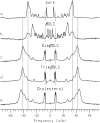
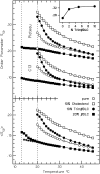
Cyclodextrins are hydrophilic molecular cages with a hydrophobic interior allowing the inclusion of water-insoluble drugs. Amphiphilic cyclodextrins obtained by appending a hydrophobic anchor were designed to improve the cell targeting of the drug-containing cavities through their liposome transportation in the organism. After insertion in model membranes, they were found to induce a lateral phase separation into a pure lipid phase and a fluid cyclodextrin-rich phase (L(CD)) with reduced acyl chain order parameters, as observed with a derivative containing a cholesterol anchor (M. Roux, R. Auzely-Velty, F. Djedaïni-Pilard, and B. Perly. 2002. Biophysical Journal, 8:813-822). We present another class of amphiphilic cyclodextrins obtained by grafting aspartic acid esterified by two lauryl chains on the oligosaccharide core via a succinyl spacer. The obtained dilauryl-beta-cyclodextrin (betaDLC) was inserted in chain perdeuterated dimyristoylphosphatidylcholine (DMPC-d54) membranes and studied by deuterium NMR ((2)H-NMR). A laterally segregated mixed phase was found to sequester three times more lipids than the cholesteryl derivative (approximately 4-5 lipids per monomer of betaDLC), and a quasipure L(CD) phase could be obtained with a 20% molar concentration of betaDLC. When cooled below the main fluid-to-gel transition of DMPC-d54 the betaDLC-rich phase stays fluid, coexisting with pure lipid in the gel state, and exhibits a sharp transition to a gel phase with frozen DMPC acyl chains at 12.5 degrees C. No lateral phase separation was observed with partially or fully methylated betaDLC, confirming that the stability of the segregated L(CD) phase was governed through hydrogen-bond-mediated intermolecular interactions between cyclodextrin headgroups at the membrane surface. As opposed to native betaDLC, the methylated derivatives were found to strongly increase the orientational order of DMPC acyl chains as the temperature reaches the membrane fluid-to-gel transition. The results are discussed in relation to the "anomalous swelling" of saturated phosphatidylcholine multilamellar membranes known to occur in the vicinity of the main fluid-to-gel transition.
Figures
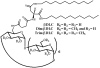

Similar articles
-
Self-assemblies of amphiphilic cyclodextrins.Eur Biophys J. 2007 Nov;36(8):861-7. doi: 10.1007/s00249-007-0207-6. Epub 2007 Jul 31. Eur Biophys J. 2007. PMID: 17665188 Review.
-
Dynamic lipid lateral segregation driven by lauryl cyclodextrin interactions at the membrane surface.Langmuir. 2013 Mar 19;29(11):3677-87. doi: 10.1021/la304524a. Epub 2013 Mar 6. Langmuir. 2013. PMID: 23409976
-
Cyclodextrin-induced lipid lateral separation in DMPC membranes: (2)H nuclear magnetic resonance study.Biophys J. 2002 Feb;82(2):813-22. doi: 10.1016/S0006-3495(02)75443-X. Biophys J. 2002. PMID: 11806923 Free PMC article.
-
Ordering of Saturated and Unsaturated Lipid Membranes near Their Phase Transitions Induced by an Amphiphilic Cyclodextrin and Cholesterol.Langmuir. 2019 Nov 5;35(44):14376-14387. doi: 10.1021/acs.langmuir.9b02082. Epub 2019 Oct 22. Langmuir. 2019. PMID: 31564102
-
Structure and functional properties of diacylglycerols in membranes.Prog Lipid Res. 1999 Jan;38(1):1-48. doi: 10.1016/s0163-7827(98)00021-6. Prog Lipid Res. 1999. PMID: 10396601 Review.
Cited by
-
Nano-Assemblies of Modified Cyclodextrins and Their Complexes with Guest Molecules: Incorporation in Nanostructured Membranes and Amphiphile Nanoarchitectonics Design.Nanomaterials (Basel). 2014 Aug 20;4(3):741-765. doi: 10.3390/nano4030741. Nanomaterials (Basel). 2014. PMID: 28344245 Free PMC article. Review.
-
Self-assemblies of amphiphilic cyclodextrins.Eur Biophys J. 2007 Nov;36(8):861-7. doi: 10.1007/s00249-007-0207-6. Epub 2007 Jul 31. Eur Biophys J. 2007. PMID: 17665188 Review.
References
-
- Binder, W. H., V. Barragan, and F. M. Menger. 2003. Domains and rafts in lipid membranes. Angew. Chem. Int. Ed. Engl. 42:5802–5827. - PubMed
-
- Devaux, P. F., and R. Morris. 2004. Transmembrane asymmetry and lateral domains in biological membranes. Traffic. 5:241–246. - PubMed
-
- Silvius, J. R. 2005. Partitioning of membrane molecules between raft and non-raft domains: insights from model-membrane studies. Biochim. Biophys. Acta. 1746:193–202. - PubMed
-
- London, E. 2005. How principles of domain formation in model membranes may explain ambiguities concerning lipid raft formation in cells. Biochim. Biophys. Acta. 1746:203–220. - PubMed
-
- Veatch, S. L., and S. L. Keller. 2005. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta. 1746:172–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

