Solute transport in growth plate cartilage: in vitro and in vivo
- PMID: 17496046
- PMCID: PMC1913140
- DOI: 10.1529/biophysj.106.097675
Solute transport in growth plate cartilage: in vitro and in vivo
Abstract
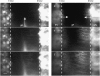
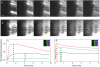
Bone elongation originates from cartilaginous discs (growth plates) at both ends of a growing bone. Here chondrocytes proliferate and subsequently enlarge (hypertrophy), laying down a matrix that serves as the scaffolding for subsequent bone matrix deposition. Because cartilage is generally avascular, all nutrients, oxygen, signaling molecules, and waste must be transported relatively long distances through the tissue for it to survive and function. Here we examine the transport properties of growth plate cartilage. Ex vivo, fluorescence photobleaching recovery methods are used in tissue explants. In vivo, multiphoton microscopy is used to image through an intact perichondrium and into the cartilage of anesthetized mice. Systemically introduced fluorescent tracers are monitored directly as they move from the vasculature into the cartilage. We demonstrate the existence of a relatively permissive region at the midplane of the growth plate, where chondrocytes transition from late proliferative to early hypertrophic stages and where paracrine communication is known to occur between chondrocytes and cells in the surrounding perichondrium. Transport in the living mouse is also significantly affected by fluid flow from the two chondro-osseus junctions, presumably resulting from a pressure difference between the bone vasculature and the cartilage.
Figures





Similar articles
-
Temperature alters solute transport in growth plate cartilage measured by in vivo multiphoton microscopy.J Appl Physiol (1985). 2009 Jun;106(6):2016-25. doi: 10.1152/japplphysiol.00295.2009. Epub 2009 Apr 16. J Appl Physiol (1985). 2009. PMID: 19372302 Free PMC article.
-
Imaging IGF-I uptake in growth plate cartilage using in vivo multiphoton microscopy.J Appl Physiol (1985). 2017 Nov 1;123(5):1101-1109. doi: 10.1152/japplphysiol.00645.2017. Epub 2017 Aug 10. J Appl Physiol (1985). 2017. PMID: 28798204 Free PMC article.
-
Condensation of hypertrophic chondrocytes at the chondro-osseous junction of growth plate cartilage in Yucatan swine: relationship to long bone growth.Am J Anat. 1989 Dec;186(4):346-58. doi: 10.1002/aja.1001860404. Am J Anat. 1989. PMID: 2589219
-
Fate of growth plate hypertrophic chondrocytes: death or lineage extension?Dev Growth Differ. 2015 Feb;57(2):179-92. doi: 10.1111/dgd.12203. Epub 2015 Feb 24. Dev Growth Differ. 2015. PMID: 25714187 Review.
-
The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification.J Endocrinol. 2011 Nov;211(2):109-21. doi: 10.1530/JOE-11-0048. Epub 2011 Jun 3. J Endocrinol. 2011. PMID: 21642379 Review.
Cited by
-
The Actions of IGF-1 in the Growth Plate and Its Role in Postnatal Bone Elongation.Curr Osteoporos Rep. 2020 Jun;18(3):210-227. doi: 10.1007/s11914-020-00570-x. Curr Osteoporos Rep. 2020. PMID: 32415542 Free PMC article. Review.
-
Temperature alters solute transport in growth plate cartilage measured by in vivo multiphoton microscopy.J Appl Physiol (1985). 2009 Jun;106(6):2016-25. doi: 10.1152/japplphysiol.00295.2009. Epub 2009 Apr 16. J Appl Physiol (1985). 2009. PMID: 19372302 Free PMC article.
-
Phosphorescent nanoparticles for quantitative measurements of oxygen profiles in vitro and in vivo.Biomaterials. 2012 Mar;33(9):2710-22. doi: 10.1016/j.biomaterials.2011.11.048. Epub 2012 Jan 10. Biomaterials. 2012. PMID: 22240511 Free PMC article.
-
Exercise mitigates the stunting effect of cold temperature on limb elongation in mice by increasing solute delivery to the growth plate.J Appl Physiol (1985). 2010 Dec;109(6):1869-79. doi: 10.1152/japplphysiol.01022.2010. Epub 2010 Oct 7. J Appl Physiol (1985). 2010. PMID: 20930127 Free PMC article.
-
Histone deacetylase 3 supports endochondral bone formation by controlling cytokine signaling and matrix remodeling.Sci Signal. 2016 Aug 9;9(440):ra79. doi: 10.1126/scisignal.aaf3273. Sci Signal. 2016. PMID: 27507649 Free PMC article.
References
-
- Breur, G. J., B. A. VanEnkevort, C. E. Farnum, and N. J. Wilsman. 1991. Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone growth in growth plates. J. Orthop. Res. 9:348–359. - PubMed
-
- Wilsman, N. J., C. E. Farnum, E. M. Leiferman, M. Fry, and C. Barreto. 1996. Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics. J. Orthop. Res. 14:927–936. - PubMed
-
- Karsenty, G., and E. F. Wagner. 2002. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell. 2:389–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

