Effects of monovalent anions of the hofmeister series on DPPC lipid bilayers Part I: swelling and in-plane equations of state
- PMID: 17496051
- PMCID: PMC1948043
- DOI: 10.1529/biophysj.106.094482
Effects of monovalent anions of the hofmeister series on DPPC lipid bilayers Part I: swelling and in-plane equations of state
Abstract
Aiming to improve understanding of the mechanisms behind specific anion effects in biological systems we have studied the effects of sodium salts of simple monovalent anions belonging to the Hofmeister series on the bilayers of the zwitterionic lipid 1,2-dipalmitoyl-sn-glycero-3-phosphocholine using small-angle x-ray scattering and the osmotic stress technique. NaCl, NaBr, NaNO(3), NaI, and NaSCN were used in this investigation. The electrolytes were found to swell the bilayers and to increase the area per lipid headgroup at each value of the osmotic pressure, suggesting the association of anions with the bilayer-lipid interfaces. The effects follow the Hofmeister series with SCN(-) inducing the most pronounced changes. "Ion competition" experiments with mixed NaI/NaCl solutions at total salinity 0.1 and 0.5 M revealed that the effect of ions on the lipid equation-of-state is roughly linear at low concentrations, but strongly nonlinear at high concentrations. The experimental results are fitted in a companion article to provide "binding" or "partitioning" constants of anions in the lipid bilayers.
Figures

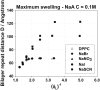


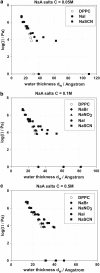
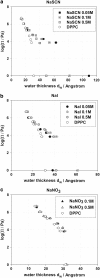

References
-
- Kunz, W., J. Henle, and B. W. Ninham. 2004. About the science of the effect of salts: Franz Hofmeister's historical papers. Curr. Opin. Colloid Int. Sci. 9:19–37.
-
- Collins, K. D., and M. W. Washabaugh. 1985. The Hofmeister effect and the behaviour of water at interfaces. Q. Rev. Biophys. 18:323–422. - PubMed
-
- Cacace, M. G., E. M. Landau, and J. J. Ramsden. 1997. The Hofmeister series: salt and solvent effects on interfacial phenomena. Q. Rev. Biophys. 30:241–277. - PubMed
-
- Curr. Op. Colloid Int. Sci. 2004. 9:1–197.
-
- Melander, W., and C. Horvath. 1977. Salt effects on hydrophobic interactions in precipitation and chromatography of proteins: an interpretation of the lyotropic series. Arch. Biochem. Biophys. 183:200–215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

