Secondary and tertiary structure elasticity of titin Z1Z2 and a titin chain model
- PMID: 17496052
- PMCID: PMC1948054
- DOI: 10.1529/biophysj.107.105528
Secondary and tertiary structure elasticity of titin Z1Z2 and a titin chain model
Abstract
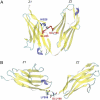
The giant protein titin, which is responsible for passive elasticity in muscle fibers, is built from approximately 300 regular immunoglobulin-like (Ig) domains and FN-III repeats. While the soft elasticity derived from its entropic regions, as well as the stiff mechanical resistance derived from the unfolding of the secondary structure elements of Ig- and FN-III domains have been studied extensively, less is known about the mechanical elasticity stemming from the orientation of neighboring domains relative to each other. Here we address the dynamics and energetics of interdomain arrangement of two adjacent Ig-domains of titin, Z1, and Z2, using molecular dynamics (MD) simulations. The simulations reveal conformational flexibility, due to the domain-domain geometry, that lends an intermediate force elasticity to titin. We employ adaptive biasing force MD simulations to calculate the energy required to bend the Z1Z2 tandem open to identify energetically feasible interdomain arrangements of the Z1 and Z2 domains. The finding is cast into a stochastic model for Z1Z2 interdomain elasticity that is generalized to a multiple domain chain replicating many Z1Z2-like units and representing a long titin segment. The elastic properties of this chain suggest that titin derives so-called tertiary structure elasticity from bending and twisting of its domains. Finally, we employ steered molecular dynamics simulations to stretch individual Z1 and Z2 domains and characterize the so-called secondary structure elasticity of the two domains. Our study suggests that titin's overall elastic response at weak force stems from a soft entropic spring behavior (not described here), from tertiary structure elasticity with an elastic spring constant of approximately 0.001-1 pN/A and, at strong forces, from secondary structure elasticity.
Figures




References
-
- Wang, K. 1996. Titin/connectin and nebulin: giant protein ruler of muscle structure and function. Adv. Biophys. 33:123–134. - PubMed
-
- Erickson, H. 1997. Stretching single protein modules: titin is a weird spring. Science. 276:1090–1093. - PubMed
-
- Maruyama, K. 1997. Connectin/titin, a giant elastic protein of muscle. FASEB J. 11:341–345. - PubMed
-
- Linke, W. 2000. Stretching molecular springs: elasticity of titin filaments in vertebrate striated muscle. Histol. Histopath. 15:799–811. - PubMed
-
- Tskhovrebova, L., and J. Trinick. 2003. Titin: properties and family relationships. Nat. Rev. Mol. Cell Biol. 4:679–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

