Low concentrations of bile salts induce stress responses and reduce motility in Bacillus cereus ATCC 14579 [corrected]
- PMID: 17496091
- PMCID: PMC1951874
- DOI: 10.1128/JB.00239-07
Low concentrations of bile salts induce stress responses and reduce motility in Bacillus cereus ATCC 14579 [corrected]
Erratum in
- J Bacteriol. 2007 Sep;189(18):6741
Abstract
Tolerance to bile salts was investigated in forty Bacillus cereus strains, including 17 environmental isolates, 11 dairy isolates, 3 isolates from food poisoning outbreaks, and 9 other clinical isolates. Growth of all strains was observed at low bile salt concentrations, but no growth was observed on LB agar plates containing more than 0.005% bile salts. Preincubation of the B. cereus type strain, ATCC 14579, in low levels of bile salts did not increase tolerance levels. B. cereus ATCC 14579 was grown to mid-exponential growth phase and shifted to medium containing bile salts (0.005%). Global expression patterns were determined by hybridization of total cDNA to a 70-mer oligonucleotide microarray. A general stress response and a specific response to bile salts were observed. The general response was similar to that observed in cultures grown in the absence of bile salts but at a higher (twofold) cell density. Up-regulation of several putative multidrug exporters and transcriptional regulators and down-regulation of most motility genes were observed as part of the specific response. Motility experiments in soft agar showed that motility decreased following bile salts exposure, in accordance with the transcriptional data. Genes encoding putative virulence factors were either unaffected or down-regulated.
Figures
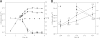


References
-
- Agaisse, H., M. Gominet, O. A. Økstad, A. B. Kolstø, and D. Lereclus. 1999. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol. Microbiol. 32:1043-1053. - PubMed
-
- Agata, N., M. Ohta, M. Mori, and M. Isobe. 1995. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol. Lett. 129:17-19. - PubMed
-
- Alekshun, M. N., and S. B. Levy. 1999. The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 7:410-413. - PubMed
-
- Alekshun, M. N., S. B. Levy, T. R. Mealy, B. A. Seaton, and J. F. Head. 2001. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 angstrom resolution. Nat. Struct. Biol. 8:710-714. - PubMed
-
- Andersson, A., P. E. Granum, and U. Ronner. 1998. The adhesion of Bacillus cereus spores to epithelial cells might be an additional virulence mechanism. Int. J. Food Microbiol. 39:93-99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

