The Haemophilus influenzae hFbpABC Fe3+ transporter: analysis of the membrane permease and development of a gallium-based screen for mutants
- PMID: 17496104
- PMCID: PMC1951847
- DOI: 10.1128/JB.00145-07
The Haemophilus influenzae hFbpABC Fe3+ transporter: analysis of the membrane permease and development of a gallium-based screen for mutants
Abstract
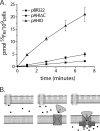
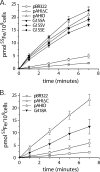
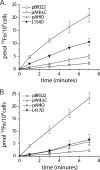
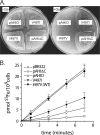
The obligate human pathogen Haemophilus influenzae utilizes a siderophore-independent (free) Fe(3+) transport system to obtain this essential element from the host iron-binding protein transferrin. The hFbpABC transporter is a binding protein-dependent ABC transporter that functions to shuttle (free) Fe(3+) through the periplasm and across the inner membrane of H. influenzae. This investigation focuses on the structure and function of the hFbpB membrane permease component of the transporter, a protein that has eluded prior characterization. Based on multiple-sequence alignments between permease orthologs, a series of site-directed mutations targeted at residues within the two conserved permease motifs were generated. The hFbpABC transporter was expressed in a siderophore-deficient Escherichia coli background, and effects of mutations were analyzed using growth rescue and radiolabeled (55)Fe(3+) transport assays. Results demonstrate that mutation of the invariant glycine (G418A) within motif 2 led to attenuated transport activity, while mutation of the invariant glycine (G155A/V/E) within motif 1 had no discernible effect on activity. Individual mutations of well-conserved leucines (L154D and L417D) led to attenuated and null transport activities, respectively. As a complement to site-directed methods, a mutant screen based on resistance to the toxic iron analog gallium, an hFbpABC inhibitor, was devised. The screen led to the identification of several significant hFbpB mutations; V497I, I174F, and S475I led to null transport activities, while S146Y resulted in attenuated activity. Significant residues were mapped to a topological model of the hFbpB permease, and the implications of mutations are discussed in light of structural and functional data from related ABC transporters.
Figures


Similar articles
-
The hFbpABC transporter from Haemophilus influenzae functions as a binding-protein-dependent ABC transporter with high specificity and affinity for ferric iron.J Bacteriol. 2004 Sep;186(18):6220-9. doi: 10.1128/JB.186.18.6220-6229.2004. J Bacteriol. 2004. PMID: 15342592 Free PMC article.
-
The role of vicinal tyrosine residues in the function of Haemophilus influenzae ferric-binding protein A.Biochem J. 2010 Nov 15;432(1):57-64. doi: 10.1042/BJ20101043. Biochem J. 2010. PMID: 20799927
-
Brucella abortus tandem repeated ATP-binding proteins, BapA and BapB, homologs of haemophilus influenzae LktB, are not necessary for intracellular survival.Microb Pathog. 2000 Oct;29(4):245-53. doi: 10.1006/mpat.2000.0389. Microb Pathog. 2000. PMID: 10993743
-
Tripartite ATP-Independent Periplasmic (TRAP) Transporters and Tripartite Tricarboxylate Transporters (TTT): From Uptake to Pathogenicity.Front Cell Infect Microbiol. 2018 Feb 12;8:33. doi: 10.3389/fcimb.2018.00033. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29479520 Free PMC article. Review.
-
Fate of ferrisiderophores after import across bacterial outer membranes: different iron release strategies are observed in the cytoplasm or periplasm depending on the siderophore pathways.Amino Acids. 2013 May;44(5):1267-77. doi: 10.1007/s00726-013-1468-2. Epub 2013 Feb 27. Amino Acids. 2013. PMID: 23443998 Review.
Cited by
-
Moonlighting of Haemophilus influenzae heme acquisition systems contributes to the host airway-pathogen interplay in a coordinated manner.Virulence. 2019 Dec;10(1):315-333. doi: 10.1080/21505594.2019.1596506. Virulence. 2019. PMID: 30973092 Free PMC article.
-
Kinetics and mechanism of exogenous anion exchange in FeFbpA-NTA: significance of periplasmic anion lability and anion binding activity of ferric binding protein A.J Biol Inorg Chem. 2010 Feb;15(2):237-48. doi: 10.1007/s00775-009-0589-2. Epub 2009 Oct 8. J Biol Inorg Chem. 2010. PMID: 19813031
-
Stimulation of expression of a silica-induced protein (Sip) in Thermus thermophilus by supersaturated silicic acid.Appl Environ Microbiol. 2009 Apr;75(8):2406-13. doi: 10.1128/AEM.02387-08. Epub 2009 Feb 20. Appl Environ Microbiol. 2009. PMID: 19233950 Free PMC article.
-
Genome analysis of Moraxella catarrhalis strain BBH18, [corrected] a human respiratory tract pathogen.J Bacteriol. 2010 Jul;192(14):3574-83. doi: 10.1128/JB.00121-10. Epub 2010 May 7. J Bacteriol. 2010. PMID: 20453089 Free PMC article.
-
Ga3+ as a mechanistic probe in Fe3+ transport: characterization of Ga3+ interaction with FbpA.J Biol Inorg Chem. 2008 Aug;13(6):887-98. doi: 10.1007/s00775-008-0376-5. Epub 2008 May 7. J Biol Inorg Chem. 2008. PMID: 18461372
References
-
- Adhikari, P., S. D. Kirby, A. J. Nowalk, K. L. Veraldi, A. B. Schryvers, and T. A. Mietzner. 1995. Biochemical characterization of a Haemophilus influenzae periplasmic iron transport operon. J. Biol. Chem. 270:25142-25149. - PubMed
-
- Albrecht-Gary, A. M., and A. L. Crumbliss. 1998. The coordination chemistry of siderophores: thermodynamics and kinetics of iron chelation and release. In A. Sigel and H. Sigel (ed.), Metal ions in biological systems, vol. 35. M. Dekker, Inc., New York, NY. - PubMed
-
- Ames, G. F.-L. 1986. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu. Rev. Biochem. 55:397-425. - PubMed
-
- Ames, G. F., C. S. Mimura, S. R. Holbrook, and V. Shyamala. 1992. Traffic ATPases: a superfamily of transport proteins operating from Escherichia coli to humans. Adv. Enzymol. Relat. Areas Mol. Biol. 65:1-47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

