Endoglin inhibits prostate cancer motility via activation of the ALK2-Smad1 pathway
- PMID: 17496924
- PMCID: PMC2199239
- DOI: 10.1038/sj.onc.1210533
Endoglin inhibits prostate cancer motility via activation of the ALK2-Smad1 pathway
Abstract
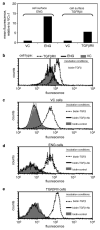
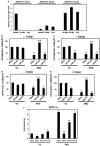
Endoglin is a transforming growth factor beta (TGFbeta) superfamily auxiliary receptor. We had previously shown that it suppressed prostate cancer (PCa) cell motility, and that its expression was lost during PCa progression. The mechanism by which endoglin inhibits PCa cell motility is unknown. Here we demonstrate that endoglin abrogates TGFbeta-mediated cell motility, but does not alter cell surface binding of TGFbeta. By measuring Smad-specific phosphorylation and Smad-responsive promoter activity, endoglin was shown to constitutively activate Smad1, with little-to-no effect upon Smad3. Knockdown of Smad1 increased motility and abrogated endoglin's effects. As type I activin receptor-like kinases (ALKs) are necessary for Smad activation, we went on to show that knockdown of ALK2, but not TGFbetaRI (ALK5), abrogated endoglin-mediated decreases in cell motility and constitutively active ALK2 was sufficient to restore a low-motility phenotype in endoglin deficient cells. These findings provide the first evidence that endoglin decreases PCa cell motility through activation of the ALK2-Smad1 pathway.
Figures






Similar articles
-
Genistein induces phenotypic reversion of endoglin deficiency in human prostate cancer cells.Mol Pharmacol. 2008 Jan;73(1):235-42. doi: 10.1124/mol.107.038935. Epub 2007 Oct 19. Mol Pharmacol. 2008. PMID: 17951357
-
Endoglin phosphorylation by ALK2 contributes to the regulation of prostate cancer cell migration.Carcinogenesis. 2010 Mar;31(3):359-66. doi: 10.1093/carcin/bgp217. Epub 2009 Sep 7. Carcinogenesis. 2010. PMID: 19736306 Free PMC article.
-
Endoglin-mediated suppression of prostate cancer invasion is regulated by activin and bone morphogenetic protein type II receptors.PLoS One. 2013 Aug 13;8(8):e72407. doi: 10.1371/journal.pone.0072407. eCollection 2013. PLoS One. 2013. PMID: 23967299 Free PMC article.
-
Hereditary hemorrhagic telangiectasia, a vascular dysplasia affecting the TGF-beta signaling pathway.Clin Med Res. 2006 Mar;4(1):66-78. doi: 10.3121/cmr.4.1.66. Clin Med Res. 2006. PMID: 16595794 Free PMC article. Review.
-
The role of the TGF-β coreceptor endoglin in cancer.ScientificWorldJournal. 2010 Dec 14;10:2367-84. doi: 10.1100/tsw.2010.230. ScientificWorldJournal. 2010. PMID: 21170488 Free PMC article. Review.
Cited by
-
MEK4 function, genistein treatment, and invasion of human prostate cancer cells.J Natl Cancer Inst. 2009 Aug 19;101(16):1141-55. doi: 10.1093/jnci/djp227. Epub 2009 Jul 28. J Natl Cancer Inst. 2009. PMID: 19638505 Free PMC article. Clinical Trial.
-
Heat shock protein 27 regulates human prostate cancer cell motility and metastatic progression.Oncotarget. 2014 May 15;5(9):2648-63. doi: 10.18632/oncotarget.1917. Oncotarget. 2014. PMID: 24798191 Free PMC article.
-
Identification of Endoglin as an epigenetically regulated tumour-suppressor gene in lung cancer.Br J Cancer. 2015 Sep 15;113(6):970-8. doi: 10.1038/bjc.2015.302. Epub 2015 Sep 1. Br J Cancer. 2015. PMID: 26325105 Free PMC article.
-
A kinase shRNA screen links LATS2 and the pRB tumor suppressor.Genes Dev. 2011 Apr 15;25(8):814-30. doi: 10.1101/gad.2000211. Genes Dev. 2011. PMID: 21498571 Free PMC article.
-
ALK1Fc Suppresses the Human Prostate Cancer Growth in in Vitro and in Vivo Preclinical Models.Front Cell Dev Biol. 2017 Dec 5;5:104. doi: 10.3389/fcell.2017.00104. eCollection 2017. Front Cell Dev Biol. 2017. PMID: 29259971 Free PMC article.
References
-
- Attisano L, Carcamo J, Ventura F, Weis FM, Massague J, Wrana JL. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 1993;75:671–680. - PubMed
-
- Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem. 1999;274:584–594. - PubMed
-
- Bergan R, Hakim F, Schwartz GN, Kyle E, Cepada R, Szabo JM, et al. Electroporation of synthetic oligodeoxy-nucleotides: a novel technique for ex vivo bone marrow purging. Blood. 1996a;88:731–741. - PubMed
-
- Bergan R, Kyle E, Nguyen P, Trepel J, Ingui C, Neckers L. Genistein-stimulated adherence of prostate cancer cells is associated with the binding of focal adhesion kinase to beta-1-integrin. Clin Exp Metastasis. 1996b;14:389–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

