Distinction between pore assembly by staphylococcal alpha-toxin versus leukotoxins
- PMID: 17497023
- PMCID: PMC1847480
- DOI: 10.1155/2007/25935
Distinction between pore assembly by staphylococcal alpha-toxin versus leukotoxins
Abstract
The staphylococcal bipartite leukotoxins and the homoheptameric alpha-toxin belong to the same family of beta-barrel pore-forming toxins despite slight differences. In the alpha-toxin pore, the N-terminal extremity of each protomer interacts as a deployed latch with two consecutive protomers in the vicinity of the pore lumen. N-terminal extremities of leukotoxins as seen in their three-dimensional structures are heterogeneous in length and take part in the beta-sandwich core of soluble monomers. Hence, the interaction of these N-terminal extremities within structures of adjacent monomers is questionable. We show here that modifications of their N-termini by two different processes, using fusion with glutathione S-transferase (GST) and bridging of the N-terminal extremity to the adjacent beta-sheet via disulphide bridges, are not deleterious for biological activity. Therefore, bipartite leukotoxins do not need a large extension of their N-terminal extremities to form functional pores, thus illustrating a microheterogeneity of the structural organizations between bipartite leukotoxins and alpha-toxin.
Figures







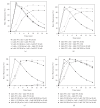
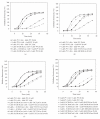


References
-
- Prévost G. Toxins in Staphylococcus aureus pathogenesis. In: Proft T, editor. Microbial Toxins. Molecular and Cellular Biology. Norfolk, UK: Horizon Bioscience; 2005. pp. 243–284.
-
- Ferreras M, Höper F, Dalla Serra M, Colin DA, Prévost G, Menestrina G. The interaction of Staphylococcus aureus bi-component γ-hemolysins and leucocidins with cells and lipid membranes. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1998;1414(1-2):108–126. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

