A comparative NMR study of the polypeptide backbone dynamics of hemoglobin in the deoxy and carbonmonoxy forms
- PMID: 17497935
- PMCID: PMC2533159
- DOI: 10.1021/bi602654u
A comparative NMR study of the polypeptide backbone dynamics of hemoglobin in the deoxy and carbonmonoxy forms
Abstract
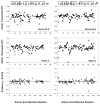
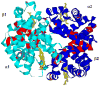
Model-free-based NMR dynamics studies have been undertaken for polypeptide backbone amide N-H bond vectors for both the deoxy and carbonmonoxy forms of chain-specific, isotopically (15N and 2H) labeled tetrameric hemoglobin (Hb) using 15N-relaxation parameters [longitudinal relaxation rate (R1), transverse relaxation rate (R2), and heteronuclear nuclear Overhauser effect (NOE)] measured at two temperatures (29 and 34 degrees C) and two magnetic field strengths (11.7 and 14.1 T). In both deoxy and carbonmonoxy forms of human normal adult hemoglobin (Hb A), the amide N-H bonds of most amino acid residues are rigid on the fast time scale (nanosecond to picosecond), except for the loop regions and certain helix-helix connections. Although rigid in deoxy-Hb A, beta146His has been found to be free from restriction of its backbone motions in the CO form, presumably due to the rupture of its hydrogen bond/salt bridge network. We now have direct dynamics evidence for this structural transition of Hb in solution. While remarkably flexible in the deoxy state, alpha31Arg and beta123Thr, neighbors in the intradimer (alpha1beta1) interface, exhibit stiffening upon CO binding. These findings imply a role for alpha31Arg and beta123Thr in the intradimer communication but contradict the results from X-ray crystallography. We have also found that there is considerable flexibility in the intradimer (alpha1beta1) interface (i.e., B, G, and H helices and the GH corner) and possible involvement of several amino acid residues (e.g., alpha31Arg, beta3Leu, beta41Phe, beta123Thr, and beta146His) in the allosteric pathway. Several amino acid residues at the intradimer interfaces, such as beta109Val, appear to be involved in possible conformational exchange processes. The dynamic picture derived from the present study provides new insights into the traditional description of the stereochemical mechanism for the cooperative oxygenation of Hb A based on X-ray crystallographic results.
Figures


References
-
- Dickerson RE, Geis I. Hemoglobin: structure, function, evolution, and pathology. Benjamin-Cummings; Menlo Park, CA: 1983.
-
- Perutz MF. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970;228:726–739. - PubMed
-
- Silva MM, Rogers PH, Arnone A. A third quaternary structure of human hemoglobin A at 1.7 A resolution. J Bio Chem. 1992;267:17248–56. - PubMed
-
- Safo MK, Abraham DJ. The enigma of the liganded hemoglobin end state: a novel quaternary structure of human carbonmonoxy hemoglobin. Biochemistry. 2005;44:8347–59. - PubMed
-
- Kavanaugh JS, Rogers PH, Arnone A. Crystallographic evidence for a new ensemble of ligand-induced allosteric transitions in hemoglobin: the T-to-Thigh quaternary transitions. Biochemistry. 2005;44:6101–6121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

