Mast cell tryptases and chymases in inflammation and host defense
- PMID: 17498057
- PMCID: PMC2275918
- DOI: 10.1111/j.1600-065X.2007.00509.x
Mast cell tryptases and chymases in inflammation and host defense
Abstract
Tryptases and chymases are the major proteins stored and secreted by mast cells. The types, amounts, and properties of these serine peptidases vary by mast cell subtype, tissue, and mammal of origin. Membrane-anchored gamma-tryptases are tryptic, prostasin-like, type I peptidases that remain membrane attached on release and act locally. Soluble tryptases, including their close relatives, mastins, form inhibitor-resistant oligomers that act more remotely. Befitting their greater destructive potential, chymases are quickly inhibited after release, although some gain protection by associating with proteoglycans. Most chymase-like enzymes, including mast cell cathepsin G, hydrolyze chymotryptic substrates, an uncommon capability in the proteome. Some rodent chymases, however, have mutations resulting in elastolytic activity. Secreted tryptases and chymases promote inflammation, matrix destruction, and tissue remodeling by several mechanisms, including destroying procoagulant, matrix, growth, and differentiation factors and activating proteinase-activated receptors, urokinase, metalloproteinases, and angiotensin. They also modulate immune responses by hydrolyzing chemokines and cytokines. At least one chymase protects mice from intestinal worms. Tryptases and chymases can also oppose inflammation by inactivating allergens and neuropeptides causing inflammation and bronchoconstriction. Thus, like mast cells themselves, mast cell serine peptidases play multiple roles in host defense, and any accounting of benefit versus harm is necessarily context specific.
Figures
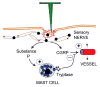
References
-
- Pallaoro M, Fejzo MS, Shayesteh L, Blount JL, Caughey GH. Characterization of genes encoding known and novel human mast cell tryptases on chromosome 16p13.3. J Biol Chem. 1999;274:3355–3362. - PubMed
-
- Caughey GH, et al. Characterization of human γ-tryptases, novel members of the chromosome 16p mast cell tryptase and prostasin gene families. J Immunol. 2000;164:6566–6575. - PubMed
-
- Caughey GH. Genetic insights into mast cell chymase and tryptase function. Clin Exp All Rev. 2004;4:96–101.
-
- Wong GW, et al. Identification of a new member of the tryptase family of mouse and human mast cell proteases which possesses a novel COOH-terminal hydrophobic extension. J Biol Chem. 1999;274:30784–30793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

