Evolution of pigment synthesis pathways by gene and genome duplication in fish
- PMID: 17498288
- PMCID: PMC1890551
- DOI: 10.1186/1471-2148-7-74
Evolution of pigment synthesis pathways by gene and genome duplication in fish
Abstract
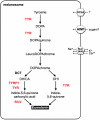
Background: Coloration and color patterning belong to the most diverse phenotypic traits in animals. Particularly, teleost fishes possess more pigment cell types than any other group of vertebrates. As the result of an ancient fish-specific genome duplication (FSGD), teleost genomes might contain more copies of genes involved in pigment cell development than tetrapods. No systematic genomic inventory allowing to test this hypothesis has been drawn up so far for pigmentation genes in fish, and almost nothing is known about the evolution of these genes in different fish lineages.
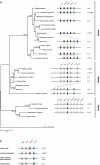
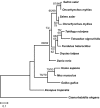
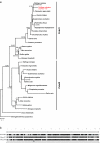
Results: Using a comparative genomic approach including phylogenetic reconstructions and synteny analyses, we have studied two major pigment synthesis pathways in teleost fish, the melanin and the pteridine pathways, with respect to different types of gene duplication. Genes encoding three of the four enzymes involved in the synthesis of melanin from tyrosine have been retained as duplicates after the FSGD. In the pteridine pathway, two cases of duplicated genes originating from the FSGD as well as several lineage-specific gene duplications were observed. In both pathways, genes encoding the rate-limiting enzymes, tyrosinase and GTP-cyclohydrolase I (GchI), have additional paralogs in teleosts compared to tetrapods, which have been generated by different modes of duplication. We have also observed a previously unrecognized diversity of gchI genes in vertebrates. In addition, we have found evidence for divergent resolution of duplicated pigmentation genes, i.e., differential gene loss in divergent teleost lineages, particularly in the tyrosinase gene family.
Conclusion: Mainly due to the FSGD, teleost fishes apparently have a greater repertoire of pigment synthesis genes than any other vertebrate group. Our results support an important role of the FSGD and other types of duplication in the evolution of pigmentation in fish.
Figures





References
-
- Fujii R. Coloration and chromatophores. In: Evans DH, editor. The Physiology of Fishes. Boca Raton, Florida: CRC Press; 1993. pp. 535–562.
-
- Bagnara JT. Comparative anatomy and physiology of pigment cells in nonmammalian tissues. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Ortonne JP, editor. The Pigmentary System: Physiology and Pathophysiology. New York: Oxford University Press; 1998. pp. 9–40.
-
- Odenthal J, Rossnagel K, Haffter P, Kelsh RN, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Mullins MC, Nusslein-Volhard C. Mutations affecting xanthophore pigmentation in the zebrafish, Danio rerio. Development. 1996;123:391–8. - PubMed
-
- Dutton KA, Pauliny A, Lopes SS, Elworthy S, Carney TJ, Rauch J, Geisler R, Haffter P, Kelsh RN. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development. 2001;128:4113–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

