Cardiac IK1 underlies early action potential shortening during hypoxia in the mouse heart
- PMID: 17498734
- PMCID: PMC2082127
- DOI: 10.1016/j.yjmcc.2007.04.002
Cardiac IK1 underlies early action potential shortening during hypoxia in the mouse heart
Abstract
It is established that prolonged hypoxia leads to activation of K(ATP) channels and action potential (AP) shortening, but the mechanisms behind the early phase of metabolic stress remain controversial. Under normal conditions IK1 channels are constitutively active while K(ATP) channels are closed. Therefore, early changes in IK1 may underlie early AP shortening. This hypothesis was tested using transgenic mice with suppressed IK1 (AAA-TG). In isolated AAA-TG hearts AP shortening was delayed by approximately 24 s compared to WT hearts. In WT ventricular myocytes, blocking oxidative phosphorylation with 1 mM cyanide (CN; 28 degrees C) led to a 29% decrease in APD90 within approximately 3-5 min. The effect of CN was reversed by application of 100 microM Ba2+, a selective blocker of IK1, but not by 10 microM glybenclamide, a selective blocker of KATP channels. Accordingly, voltage-clamp experiments revealed that both CN and true hypoxia lead to early activation of IK1. In AAA-TG myocytes, neither CN nor glybenclamide or Ba2+ had any effect on AP. Further experiments showed that buffering of intracellular Ca2+ with 20 mM BAPTA prevented IK1 activation by CN, although CN still caused a 54% increase in IK1 in a Ca2+ -free bath solution. Importantly, both (i) 20 microM ruthenium red, a selective inhibitor of SR Ca2+ -release, and (ii) depleting SR by application of 10 microM ryanodine+1 mM caffeine, abolished the activation of IK1 by CN. The above data strongly argue that in the mouse heart IK1, not KATP, channels are responsible for the early AP shortening during hypoxia.
Figures
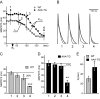
 ) leads to an early, presumably IK1-dependent, abbreviation of MAP (2;
) leads to an early, presumably IK1-dependent, abbreviation of MAP (2;
 ) followed by a further rapid MAP shortening (3;
) followed by a further rapid MAP shortening (3;
 ), presumably due to the activation of KATP channels. The time for early MAP shortening was defined as the time when MAPD75 falls by ∼10% of the Ctrl value measured just before application of N2 (1;
), presumably due to the activation of KATP channels. The time for early MAP shortening was defined as the time when MAPD75 falls by ∼10% of the Ctrl value measured just before application of N2 (1;
 ). The early phase of MAP shortening is absent in AAA-TG hearts, and only the rapid phase is observed, which starts at the same time as the KATP-dependent phase of MAP shortening in WT hearts (3;
). The early phase of MAP shortening is absent in AAA-TG hearts, and only the rapid phase is observed, which starts at the same time as the KATP-dependent phase of MAP shortening in WT hearts (3;
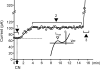
 ). B. Representative MAPs from WT (gray) and AAA-TG (black) hearts recorded at the times as indicated in A. MAP amplitudes were normalized to each other to highlight the differences in shape. C, D. MAPD75 measured at the times as indicated in A. At the time when the early MAP shortening has just begun in AAA-TG hearts (3), in WT hearts MAPD75 has been already reduced by 28%. Statistical significance is indicated compared to MAPD75 before application of N2 (1). D. The time delay (Dt) between the application of N2 and the time when MAPD75 is decreased by 10% in WT and AAA-TG hearts. n=4 for both WT AAA-TG hearts.
). B. Representative MAPs from WT (gray) and AAA-TG (black) hearts recorded at the times as indicated in A. MAP amplitudes were normalized to each other to highlight the differences in shape. C, D. MAPD75 measured at the times as indicated in A. At the time when the early MAP shortening has just begun in AAA-TG hearts (3), in WT hearts MAPD75 has been already reduced by 28%. Statistical significance is indicated compared to MAPD75 before application of N2 (1). D. The time delay (Dt) between the application of N2 and the time when MAPD75 is decreased by 10% in WT and AAA-TG hearts. n=4 for both WT AAA-TG hearts.
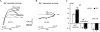



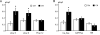
Comment in
-
IK1 and cardiac hypoxia: after the long and short QT syndromes, what else can go wrong with the inward rectifier K+ currents?J Mol Cell Cardiol. 2007 Jul;43(1):15-7. doi: 10.1016/j.yjmcc.2007.04.015. Epub 2007 Apr 29. J Mol Cell Cardiol. 2007. PMID: 17561108 Free PMC article. No abstract available.
Similar articles
-
Transgenic upregulation of IK1 in the mouse heart is proarrhythmic.Basic Res Cardiol. 2007 Sep;102(5):416-28. doi: 10.1007/s00395-007-0659-y. Epub 2007 Jun 5. Basic Res Cardiol. 2007. PMID: 17546530
-
Regulation of action potential duration under acute heat stress by I(K,ATP) and I(K1) in fish cardiac myocytes.Am J Physiol Regul Integr Comp Physiol. 2004 Feb;286(2):R405-15. doi: 10.1152/ajpregu.00500.2003. Epub 2003 Oct 30. Am J Physiol Regul Integr Comp Physiol. 2004. PMID: 14592934
-
Chronic angiotensin II stimulation in the heart produces an acquired long QT syndrome associated with IK1 potassium current downregulation.J Mol Cell Cardiol. 2007 Jan;42(1):63-70. doi: 10.1016/j.yjmcc.2006.09.019. Epub 2006 Oct 30. J Mol Cell Cardiol. 2007. PMID: 17070838
-
Regional variation of the inwardly rectifying potassium current in the canine heart and the contributions to differences in action potential repolarization.J Mol Cell Cardiol. 2015 Jul;84:52-60. doi: 10.1016/j.yjmcc.2015.04.010. Epub 2015 Apr 15. J Mol Cell Cardiol. 2015. PMID: 25889894 Free PMC article.
-
Tetramisole is a new IK1 channel agonist and exerts IK1 -dependent cardioprotective effects in rats.Pharmacol Res Perspect. 2022 Aug;10(4):e00992. doi: 10.1002/prp2.992. Pharmacol Res Perspect. 2022. PMID: 35880674 Free PMC article. Review.
Cited by
-
Puerarin: a novel antagonist to inward rectifier potassium channel (IK1).Mol Cell Biochem. 2011 Jun;352(1-2):117-23. doi: 10.1007/s11010-011-0746-0. Epub 2011 Feb 14. Mol Cell Biochem. 2011. PMID: 21327545
-
IK1 and cardiac hypoxia: after the long and short QT syndromes, what else can go wrong with the inward rectifier K+ currents?J Mol Cell Cardiol. 2007 Jul;43(1):15-7. doi: 10.1016/j.yjmcc.2007.04.015. Epub 2007 Apr 29. J Mol Cell Cardiol. 2007. PMID: 17561108 Free PMC article. No abstract available.
-
Unstable eigenmodes are possible drivers for cardiac arrhythmias.J R Soc Interface. 2011 Aug 7;8(61):1212-6. doi: 10.1098/rsif.2011.0152. Epub 2011 May 13. J R Soc Interface. 2011. PMID: 21571942 Free PMC article.
-
[Ca²⁺] i-induced augmentation of the inward rectifier potassium current (IK1) in canine and human ventricular myocardium.Pflugers Arch. 2013 Nov;465(11):1621-35. doi: 10.1007/s00424-013-1309-x. Epub 2013 Jun 27. Pflugers Arch. 2013. PMID: 23807312
-
Shear-Stress Sensitive Inwardly-Rectifying K+ Channels Regulate Developmental Retinal Angiogenesis by Vessel Regression.Cell Physiol Biochem. 2019;52(6):1569-1583. doi: 10.33594/000000109. Cell Physiol Biochem. 2019. PMID: 31145841 Free PMC article.
References
-
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8. - PubMed
-
- Fischbach PS, White A, Barrett TD, Lucchesi BR. Risk of ventricular proarrhythmia with selective opening of the myocardial sarcolemmal versus mitochondrial ATP-gated potassium channel. J Pharmacol Exp Ther. 2004;309:554–9. - PubMed
-
- Ruiz-Petrich E, de Lorenzi F, Chartier D. Role of the inward rectifier IK1 in the myocardial response to hypoxia. Cardiovasc Res. 1991;25:17–26. - PubMed
-
- Muramatsu H, Sato R, Okumura H. Early increase in K+ conductance during metabolic inhibition by cyanide in guinea pig ventricular myocytes. Nippon Ika Daigaku Zasshi. 1990;57:308–21. - PubMed
-
- Dobrev D, Wettwer E, Kortner A, Knaut M, Schuler S, Ravens U. Human inward rectifier potassium channels in chronic and postoperative atrial fibrillation. Cardiovasc Res. 2002;54:397–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

