The beta2 adrenergic receptor regulates morphine tolerance and physical dependence
- PMID: 17498818
- PMCID: PMC1989675
- DOI: 10.1016/j.bbr.2007.03.037
The beta2 adrenergic receptor regulates morphine tolerance and physical dependence
Abstract
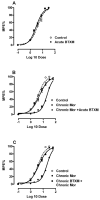
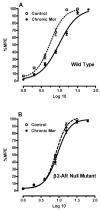
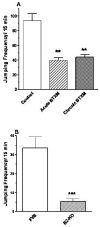
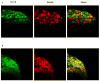
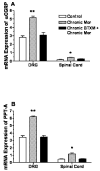
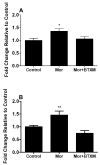
Adaptations to the chronic administration of opioids reduce the utility of these drugs in treating pain and support addiction. Recent genetics-based approaches have implicated the beta2 adrenergic receptor (beta2-AR) in controlling some of these responses. We do not know, however, whether this receptor can modulate tolerance, dependence or changes in gene expression caused by chronic opioid administration. For our studies we used C57BL/6 mice and beta2-AR knockout mice in the FVB background. Morphine dose-response relationships were established both prior to and after chronic morphine treatment. In some cases, the selective beta2-AR antagonist butoxamine was administered along with or after morphine. Physical dependence was assessed using naloxone-precipitated withdrawal. The expression of calcitonin gene related peptide (CGRP) and substance P (SP) were measured in spinal cord and dorsal root ganglion (DRG) tissues using both real-time PCR and enzyme-linked immunoassay (ELISA). Both the co-administration of butoxamine with morphine and the administration of butoxamine after chronic morphine reversed morphine tolerance. Morphine failed to cause tolerance in beta2-AR knockout mice. Physical dependence was reduced under the same circumstances. The chronic administration of butoxamine with morphine reduced or eliminated the normally observed up-regulation of CGRP and SP in spinal cord and DRG tissues. Our results suggest that the beta2-AR modulates both opioid tolerance and physical dependence. Activation of beta2-ARs appears to be required for some of the key neurochemical changes which characterize chronic opioid administration. Therefore, beta2-AR antagonists show some promise as agents to enhance chronic opioid analgesic therapy.
Figures
References
-
- Ammer H, Schulz R. Adenylyl cyclase supersensitivity in opioid-withdrawn NG108-15 hybrid cells requires Gs but is not mediated by the Gsalpha subunit. J Pharmacol Exp Ther. 1998;286:855–862. - PubMed
-
- Ammer H, Schulz R. Chronic morphine treatment increases stimulatory beta-2 adrenoceptor signaling in A431 cells stably expressing the mu opioid receptor. J Pharmacol Exp Ther. 1997;280:512–520. - PubMed
-
- Ammer H, Schulz R. Retinoic acid-induced differentiation of human neuroblastoma SH-SY5Y cells is associated with changes in the abundance of G proteins. J Neurochem. 1994;62:1310–1318. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

