The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3
- PMID: 17498976
- PMCID: PMC2724596
- DOI: 10.1016/j.joca.2007.03.008
The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3
Abstract
Objective: To determine whether the functional properties of tissue-engineered constructs cultured in a chemically-defined medium supplemented briefly with TGF-beta3 can be enhanced with the application of dynamic deformational loading.
Methods: Primary immature bovine cells (2-3 months old) were encapsulated in agarose hydrogel (2%, 30 x 10(6)cells/ml) and cultured in chemically-defined medium supplemented for the first 2 weeks with transforming growth factor beta 3 (TGF-beta3) (10 microg/ml). Physiologic deformational loading (1 Hz, 3 h/day, 10% unconfined deformation initially and tapering to 2% peak-to-peak deformation by day 42) was applied either concurrent with or after the period of TGF-beta3 supplementation. Mechanical and biochemical properties were evaluated up to day 56.
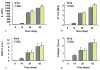
Results: Dynamic deformational loading applied concurrently with TGF-beta3 supplementation yielded significantly lower (-90%) overall mechanical properties when compared to free-swelling controls. In contrast, the same loading protocol applied after the discontinuation of the growth factor resulted in significantly increased (+10%) overall mechanical properties relative to free-swelling controls. Equilibrium modulus values reach 1306+/-79 kPa and glycosaminoglycan levels reach 8.7+/-1.6% w.w. during this 8-week period and are similar to host cartilage properties (994+/-280 kPa, 6.3+/-0.9% w.w.).
Conclusions: An optimal strategy for the functional tissue engineering of articular cartilage, particularly to accelerate construct development, may incorporate sequential application of different growth factors and applied deformational loading.
Figures





References
-
- Guilak F, Meyer BC, Ratcliffe A, Mow VC. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage. 1994;2:91–101. - PubMed
-
- Burton-Wurster N, Vernier-Singer M, Farquhar T, Lust G. Effect of compressive loading and unloading on the synthesis of total protein, proteoglycan, and fibronectin by canine cartilage explants. J Orthop Res. 1993;11:717–729. - PubMed
-
- Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108(Pt 4):1497–1508. - PubMed
-
- Lee DA, Noguchi T, Frean SP, Lees P, Bader DL. The influence of mechanical loading on isolated chondrocytes seeded in agarose constructs. Biorheology. 2000;37:149–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

