Depolarization and decreased surface expression of K+ channels contribute to NSAID-inhibition of intestinal restitution
- PMID: 17499219
- PMCID: PMC3269908
- DOI: 10.1016/j.bcp.2007.03.030
Depolarization and decreased surface expression of K+ channels contribute to NSAID-inhibition of intestinal restitution
Abstract
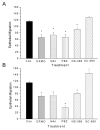
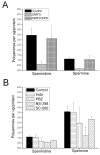
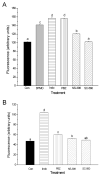
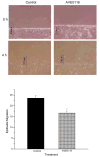
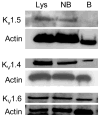
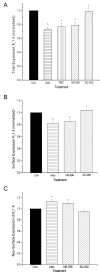
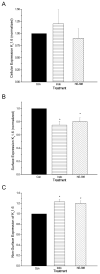
Non-steroidal anti-inflammatory drugs (NSAIDs) contribute to gastrointestinal ulcer formation by inhibiting epithelial cell migration and mucosal restitution; however, the drug-affected signaling pathways are poorly defined. We investigated whether NSAID inhibition of intestinal epithelial migration is associated with depletion of intracellular polyamines, depolarization of membrane potential (E(m)) and altered surface expression of K(+) channels. Epithelial cell migration in response to the wounding of confluent IEC-6 and IEC-Cdx2 monolayers was reduced by indomethacin (100 microM), phenylbutazone (100 microM) and NS-398 (100 microM) but not by SC-560 (1 microM). NSAID-inhibition of intestinal cell migration was not associated with depletion of intracellular polyamines. Treatment of IEC-6 and IEC-Cdx2 cells with indomethacin, phenylbutazone and NS-398 induced significant depolarization of E(m), whereas treatment with SC-560 had no effect on E(m). The E(m) of IEC-Cdx2 cells was: -38.5+/-1.8 mV under control conditions; -35.9+/-1.6 mV after treatment with SC-560; -18.8+/-1.2 mV after treatment with indomethacin; and -23.7+/-1.4 mV after treatment with NS-398. Whereas SC-560 had no significant effects on the total cellular expression of K(v)1.4 channel protein, indomethacin and NS-398 decreased not only the total cellular expression of K(v)1.4, but also the cell surface expression of both K(v)1.4 and K(v)1.6 channel subunits in IEC-Cdx2. Both K(v)1.4 and K(v)1.6 channel proteins were immunoprecipitated by K(v)1.4 antibody from IEC-Cdx2 lysates, indicating that these subunits co-assemble to form heteromeric K(v) channels. These results suggest that NSAID inhibition of epithelial cell migration is independent of polyamine-depletion, and is associated with depolarization of E(m) and decreased surface expression of heteromeric K(v)1 channels.
Figures


Similar articles
-
Inhibition of Kv channel expression by NSAIDs depolarizes membrane potential and inhibits cell migration by disrupting calpain signaling.Biochem Pharmacol. 2015 Dec 15;98(4):614-28. doi: 10.1016/j.bcp.2015.10.017. Epub 2015 Nov 7. Biochem Pharmacol. 2015. PMID: 26549367 Free PMC article.
-
Activation of K(+) channels and increased migration of differentiated intestinal epithelial cells after wounding.Am J Physiol Cell Physiol. 2002 Apr;282(4):C885-98. doi: 10.1152/ajpcell.00361.2001. Am J Physiol Cell Physiol. 2002. PMID: 11880277
-
Atractylodes macrocephala Koidz promotes intestinal epithelial restitution via the polyamine--voltage-gated K+ channel pathway.J Ethnopharmacol. 2014 Feb 27;152(1):163-72. doi: 10.1016/j.jep.2013.12.049. Epub 2014 Jan 10. J Ethnopharmacol. 2014. PMID: 24417867
-
Suppression of calpain expression by NSAIDs is associated with inhibition of cell migration in rat duodenum.Toxicology. 2017 May 15;383:1-12. doi: 10.1016/j.tox.2017.03.017. Epub 2017 Mar 22. Toxicology. 2017. PMID: 28342779 Free PMC article.
-
Cellular signaling in rapid intestinal epithelial restitution: implication of polyamines and K+ channels.Sheng Li Xue Bao. 2003 Aug 25;55(4):365-72. Sheng Li Xue Bao. 2003. PMID: 12937813 Review.
Cited by
-
Pharmacological activation of epidermal growth factor receptor signaling inhibits colitis-associated cancer in mice.Sci Rep. 2018 Jun 14;8(1):9119. doi: 10.1038/s41598-018-27353-w. Sci Rep. 2018. PMID: 29904166 Free PMC article.
-
Potassium channels in intestinal epithelial cells and their pharmacological modulation: a systematic review.Am J Physiol Cell Physiol. 2021 Apr 1;320(4):C520-C546. doi: 10.1152/ajpcell.00393.2020. Epub 2020 Dec 16. Am J Physiol Cell Physiol. 2021. PMID: 33326312 Free PMC article.
-
Nonsteroidal anti-inflammatory drugs and the risk for anastomotic failure: a report from Washington State's Surgical Care and Outcomes Assessment Program (SCOAP).JAMA Surg. 2015 Mar 1;150(3):223-8. doi: 10.1001/jamasurg.2014.2239. JAMA Surg. 2015. PMID: 25607250 Free PMC article.
-
Risk of NSAID-associated anastomosis leakage after colorectal surgery: a large-scale retrospective study using propensity score matching.Int J Colorectal Dis. 2022 May;37(5):1189-1197. doi: 10.1007/s00384-022-04160-4. Epub 2022 Apr 27. Int J Colorectal Dis. 2022. PMID: 35476135
-
Potassium Channelopathies and Gastrointestinal Ulceration.Gut Liver. 2016 Nov 15;10(6):881-889. doi: 10.5009/gnl15414. Gut Liver. 2016. PMID: 27784845 Free PMC article. Review.
References
-
- Israel LH, Koea JB, Stewart ID, Wright CL, Frankish PD. Nonsteroidal anti-inflammatory drug-induced strictures of the colon: report of a case and review of the literature. Dis Colon Rectum. 2001;44:1362–1364. - PubMed
-
- Karcher LF, Dill SG, Anderson WI, King JM. Right dorsal colitis. J Vet Intern Med. 1990;4:247–253. - PubMed
-
- Lichtenberger LM. Where is the evidence that cyclooxygenase inhibition is the primary cause of nonsteroidal anti-inflammatory drug (NSAID)-induced gastrointestinal injury? Topical injury revisited Biochem Pharmacol. 2001;61:631–637. - PubMed
-
- MacAllister CG, Morgan SJ, Borne AT, Pollet RA. Comparison of adverse effects of phenylbutazone, flunixin meglumine, and ketoprofen in horses. J Am Vet Med Assoc. 1993;202:71–77. - PubMed
-
- Whittle BJ. Gastrointestinal effects of nonsteroidal anti-inflammatory drugs. Fundam Clin Pharmacol. 2003;17:301–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

