Identification and mapping of protein kinase A binding sites in the costameric protein myospryn
- PMID: 17499862
- PMCID: PMC1955755
- DOI: 10.1016/j.bbamcr.2007.04.004
Identification and mapping of protein kinase A binding sites in the costameric protein myospryn
Abstract
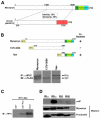
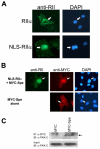
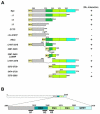
Recently we identified a novel target gene of MEF2A named myospryn that encodes a large, muscle-specific, costamere-restricted alpha-actinin binding protein. Myospryn belongs to the tripartite motif (TRIM) superfamily of proteins and was independently identified as a dysbindin-interacting protein. Dysbindin is associated with alpha-dystrobrevin, a component of the dystrophin-glycoprotein complex (DGC) in muscle. Apart from these initial findings little else is known regarding the potential function of myospryn in striated muscle. Here we reveal that myospryn is an anchoring protein for protein kinase A (PKA) (or AKAP) whose closest homolog is AKAP12, also known as gravin/AKAP250/SSeCKS. We demonstrate that myospryn co-localizes with RII alpha, a type II regulatory subunit of PKA, at the peripheral Z-disc/costameric region in striated muscle. Myospryn interacts with RII alpha and this scaffolding function has been evolutionarily conserved as the zebrafish ortholog also interacts with PKA. Moreover, myospryn serves as a substrate for PKA. These findings point to localized PKA signaling at the muscle costamere.
Figures




References
-
- Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–96. - PubMed
-
- McKinsey TA, Zhang CL, Olson EN. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci. 2002;27(1):40–7. - PubMed
-
- Paris J, Virtanen C, Lu Z, Takahashi M. Identification of MEF2-regulated genes during muscle differentiation. Physiol Genomics. 2004;20(1):143–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

