Mechanism of interaction of optimized Limulus-derived cyclic peptides with endotoxins: thermodynamic, biophysical and microbiological analysis
- PMID: 17501719
- PMCID: PMC1948972
- DOI: 10.1042/BJ20070279
Mechanism of interaction of optimized Limulus-derived cyclic peptides with endotoxins: thermodynamic, biophysical and microbiological analysis
Abstract
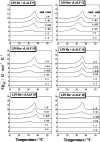
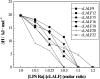
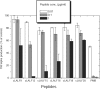
On the basis of formerly investigated peptides corresponding to the endotoxin-binding domain from LALF [Limulus anti-LPS (lipopolysaccharide) factor], a protein from Limulus polyphemus, we have designed and synthesized peptides of different lengths with the aim of obtaining potential therapeutic agents against septic shock syndrome. For an understanding of the mechanisms of action, we performed a detailed physicochemical and biophysical analysis of the interaction of rough mutant LPS with these peptides by applying FTIR (Fourier-transform infrared) spectroscopy, SAXS (small-angle X-ray scattering), calorimetric techniques [DSC (differential scanning calorimetry) and ITC (isothermal titration calorimetry)] and FFTEM (freeze-fracture transmission electron microscopy). Also, the action of the peptides on bacteria of different origin in microbial assays was investigated. Using FTIR and DSC, our results indicated a strong fluidization of the lipid A acyl chains due to peptide binding, with a decrease in the endothermic melting enthalpy change of the acyl chains down to a complete disappearance in the 1:0.5 to 1:2 [LPS]:[peptide] molar ratio range. Via ITC, it was deduced that the binding is a clearly exothermic process which becomes saturated at a 1:0.5 to 1:2 [LPS]:[peptide] molar ratio range. The results obtained with SAXS indicated a drastic change of the aggregate structures of LPS into a multilamellar stack, which was visualized in electron micrographs as hundreds of lamellar layers. This can be directly correlated with the inhibition of the LPS-induced production of tumour necrosis factor alpha in human mononuclear cells, but not with the action of the peptides on bacteria.
Figures






Similar articles
-
Cyclic antimicrobial peptides based on Limulus anti-lipopolysaccharide factor for neutralization of lipopolysaccharide.Biochem Pharmacol. 2004 Oct 1;68(7):1297-307. doi: 10.1016/j.bcp.2004.05.054. Biochem Pharmacol. 2004. PMID: 15345319
-
Biophysical characterization of the interaction of Limulus polyphemus endotoxin neutralizing protein with lipopolysaccharide.Eur J Biochem. 2004 May;271(10):2037-46. doi: 10.1111/j.1432-1033.2004.04134.x. Eur J Biochem. 2004. PMID: 15128313
-
Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin.Biol Chem. 2001 Aug;382(8):1215-25. doi: 10.1515/BC.2001.152. Biol Chem. 2001. PMID: 11592403
-
Inhibition of Lipopolysaccharide- and Lipoprotein-Induced Inflammation by Antitoxin Peptide Pep19-2.5.Front Immunol. 2018 Jul 26;9:1704. doi: 10.3389/fimmu.2018.01704. eCollection 2018. Front Immunol. 2018. PMID: 30093904 Free PMC article. Review.
-
Polymyxin and related peptide antibiotics.Annu Rev Biochem. 1977;46:723-63. doi: 10.1146/annurev.bi.46.070177.003451. Annu Rev Biochem. 1977. PMID: 197881 Review.
Cited by
-
Biophysical analysis of the interaction of the serum protein human β2GPI with bacterial lipopolysaccharide.FEBS Open Bio. 2014 May 2;4:432-40. doi: 10.1016/j.fob.2014.04.008. eCollection 2014. FEBS Open Bio. 2014. PMID: 24918058 Free PMC article.
-
Immunogenic properties of the human gut-associated archaeon Methanomassiliicoccus luminyensis and its susceptibility to antimicrobial peptides.PLoS One. 2017 Oct 5;12(10):e0185919. doi: 10.1371/journal.pone.0185919. eCollection 2017. PLoS One. 2017. PMID: 28982164 Free PMC article.
-
A Novel Hepcidin Isoform Jd-Hep from the Sin Croaker Johnius dussumieri (Cuvier, 1830): Recombinant Expression and Insights into the Antibacterial Property and Modes of Action.Mar Biotechnol (NY). 2025 Feb 19;27(2):52. doi: 10.1007/s10126-025-10426-z. Mar Biotechnol (NY). 2025. PMID: 39969620
-
Bacterial cell wall compounds as promising targets of antimicrobial agents I. Antimicrobial peptides and lipopolyamines.Curr Drug Targets. 2012 Aug;13(9):1121-30. doi: 10.2174/138945012802002410. Curr Drug Targets. 2012. PMID: 22664072 Free PMC article.
-
Antimicrobial peptides and their potential application in inflammation and sepsis.Crit Care. 2012 Dec 12;16(2):207. doi: 10.1186/cc11220. Crit Care. 2012. PMID: 22429567 Free PMC article. Review. No abstract available.
References
-
- Tobias P. S., Soldau K., Gegner J. A., Mintz D., Ulevitch R. J. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J. Biol. Chem. 1995;270:10482–10488. - PubMed
-
- Gutsmann T., Haberer N., Carroll S. F., Seydel U., Wiese A. Interaction between lipopolysaccharide (LPS), LPS-binding protein (LBP), and planar membranes. Biol. Chem. 2001;382:425–434. - PubMed
-
- Akashi S., Nagai Y., Ogata H., Oikawa M., Fukase K., Kusumoto S., Kawasaki K., Nishijima M., Hayashi S., Kimoto M., Miyake K. Human MD-2 confers on mouse Toll-like receptor 4 species-specific lipopolysaccharide recognition. Int. Immunol. 2001. 2002;13:1595–1599. - PubMed
-
- Beutler B. Endotoxin, Toll-like receptor 4, and the afferent limb of innate immunity. Curr. Opin. Microbiol. 2000;3:23–28. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

