Pharmacogenomics and the Yin/Yang actions of ginseng: anti-tumor, angiomodulating and steroid-like activities of ginsenosides
- PMID: 17502003
- PMCID: PMC1876803
- DOI: 10.1186/1749-8546-2-6
Pharmacogenomics and the Yin/Yang actions of ginseng: anti-tumor, angiomodulating and steroid-like activities of ginsenosides
Abstract
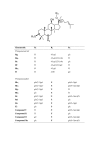
In Chinese medicine, ginseng (Panax ginseng C.A. Meyer) has long been used as a general tonic or an adaptogen to promote longevity and enhance bodily functions. It has also been claimed to be effective in combating stress, fatigue, oxidants, cancer and diabetes mellitus. Most of the pharmacological actions of ginseng are attributed to one type of its constituents, namely the ginsenosides. In this review, we focus on the recent advances in the study of ginsenosides on angiogenesis which is related to many pathological conditions including tumor progression and cardiovascular dysfunctions. Angiogenesis in the human body is regulated by two sets of counteracting factors, angiogenic stimulators and inhibitors. The 'Yin and Yang' action of ginseng on angiomodulation was paralleled by the experimental data showing angiogenesis was indeed related to the compositional ratio between ginsenosides Rg1 and Rb1. Rg1 was later found to stimulate angiogenesis through augmenting the production of nitric oxide (NO) and vascular endothelial growth factor (VEGF). Mechanistic studies revealed that such responses were mediated through the PI3K-->Akt pathway. By means of DNA microarray, a group of genes related to cell adhesion, migration and cytoskeleton were found to be up-regulated in endothelial cells. These gene products may interact in a hierarchical cascade pattern to modulate cell architectural dynamics which is concomitant to the observed phenomena in angiogenesis. By contrast, the anti-tumor and anti-angiogenic effects of ginsenosides (e.g. Rg3 and Rh2) have been demonstrated in various models of tumor and endothelial cells, indicating that ginsenosides with opposing activities are present in ginseng. Ginsenosides and Panax ginseng extracts have been shown to exert protective effects on vascular dysfunctions, such as hypertension, atherosclerotic disorders and ischemic injury. Recent work has demonstrates the target molecules of ginsenosides to be a group of nuclear steroid hormone receptors. These lines of evidence support that the interaction between ginsenosides and various nuclear steroid hormone receptors may explain the diverse pharmacological activities of ginseng. These findings may also lead to development of more efficacious ginseng-derived therapeutics for angiogenesis-related diseases.
Figures



References
-
- Hu SY. A contribution of our knowledge of ginseng. Am J Chin Med. 1977;5:1–23. - PubMed
-
- Tao HC. Sheng-Nung-Pen-Tsao-Ching. Taipei, Taiwan: Chung-Hwa; 1955.
-
- Liu CX. Introduction on research of ginseng. Information of Traditional Chinese Medicine. 1975;2:9–11.
-
- Liu CX. Pharmacology and clinic of active principles of ginseng. Chinese Traditional Herbs and Drugs. 1975;7:57.
-
- Liu CX, Xiao PG. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27–38. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

