Selection bias resulting from the requirement for prior consent in observational research: a community cohort of people with ischaemic heart disease
- PMID: 17502325
- PMCID: PMC1955022
- DOI: 10.1136/hrt.2006.111591
Selection bias resulting from the requirement for prior consent in observational research: a community cohort of people with ischaemic heart disease
Abstract
Objective: To evaluate differences between adults who consent to participate in observational research and those who do not.
Design: Prospective, population-based cohort study.
Setting: 35 randomised Irish general practices.
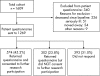
Participants: 1609 adults with ischaemic heart disease identified in 2000-1.
Intervention: Medical records search, postal questionnaire and consent form in 2005-6.
Main outcome measures: Differences in demographic and prognostic risk factors between consenters and non-consenters.
Results: At follow-up, charts were located for 1592 patients (98.9%). Questionnaires were sent to 1269 patients and 876 were returned (69%). Of these, 574 (65.5%) gave consent for participation in further research. Logistic regression identified four characteristics as independently positively predictive of consent to participation in further research among questionnaire responders: having undergone percutaneous transluminal coronary angioplasty was associated with an increased odds of consent, with an odds ratio (OR) of 1.77 (95% CI 1.09 to 2.86), as was a last recorded blood pressure <140/90 mm Hg (OR = 1.45 (1.00 to 2.09)), a last recorded total cholesterol level <5 mmol/l (OR = 1.71 (1.16 to 2.54)) and being an ex-smoker rather than a current smoker or non-smoker (OR = 1.73 (1.17 to 2.57)).
Conclusions: This research demonstrates the potential impact of consent bias in observational research on ischaemic heart disease, a disease of everyday clinical importance in Europe. It demonstrates that clinically important prognostic variables may be associated with consent preferences. Future cohorts, dependent upon prior written consent, may contain disproportionate numbers of those who have made healthy lifestyle decisions, have previously benefited from treatment or whose clinical risk factors are already well managed. As a result, the generalisability of such research may be diminished and the effects of treatments over- or underestimated.
Conflict of interest statement
Conflict of interest: None.
Comment in
-
Consent bias in research: how to avoid it.Heart. 2007 Sep;93(9):1024-5. doi: 10.1136/hrt.2007.120113. Heart. 2007. PMID: 17699171 Free PMC article.
References
-
- Medical Research Council Personal information in medical research. London: Medical Research Council, 2000
-
- Anonymous An information guide to the data proctection acts for general practitioners. Irish College of General Practitioners/National General Practice Information Technology Group 2003
-
- Data Protection Commissioner Data Protection Acts 1988 and 2003: a compendium. Dublin: Office of the Data Protection Commissioner, 2003
-
- Data Protection Commissioner Data Protection (Amendment) Act 2003: a summary guide. Dublin: The Office of the Data Protection Commissioner, 2003
-
- Anonymous Health information: a national strategy. Dublin: Department of Health and Children, 2004
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials